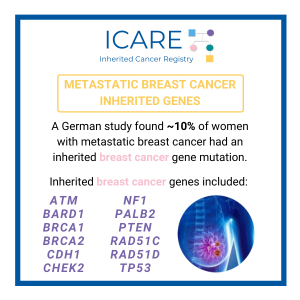
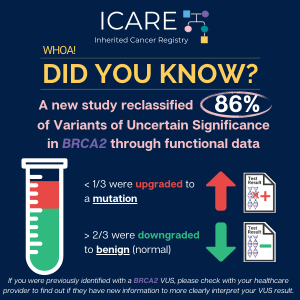
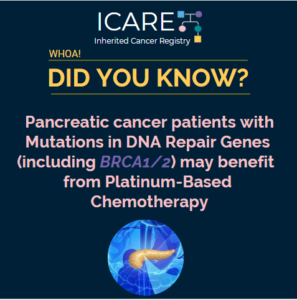
In a study of a rare type of pancreatic cancer, called pancreatic acinar cell cancer (PACC), over one third (36.7%) of a total of 49 patients with PACC had a mutation in an inherited cancer gene. The most commonly mutated gene was BRCA2 (12), and other genes included BRCA1 (1), PALB2 (2), ATM (2), and …
Tag: BRCA2
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2024-inherited-risk-in-patients-with-pancreatic-acinar-cell-carcinoma/
ICARE Newsletter Spring 2024
BRCA-Associated Prostate Cancer Treatment Updates
ICARE Newsletter Spring 2024
BRCA-Associated Prostate Cancer Treatment Updates
New studies to guide treatment strategies in men with prostate cancer and a BRCA mutation were recently published. Specifically, a recent study suggested that platinum-based chemotherapy may be as effective as PARP inhibitors for individuals with BRCA-positive metastatic castration-resistant prostate cancer.1 The study sheds light on treatment options for advanced prostate cancer patients. More recently, …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2024-brca-associated-prostate-cancer-treatment-updates/
ICARE Newsletter Spring 2024
Ask the Expert
ICARE Newsletter Spring 2024
Ask the Expert
This question was addressed by Ronald D. Alvarez, MD, MBA, Professor and Chairman of the Department of Obstetrics and Gynecology at the Vanderbilt University Medical Center in Nashville, Tennessee. He is also the current vice chair of the National Comprehensive Cancer Network (NCCN) Ovarian Cancer Treatment Guidelines and has served in multiple leadership roles in …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2024-ask-the-expert/
ICARE Newsletter Spring 2024
How Well Do Cancer Risk Management strategies Work Among BRCA Carriers
ICARE Newsletter Spring 2024
How Well Do Cancer Risk Management strategies Work Among BRCA Carriers
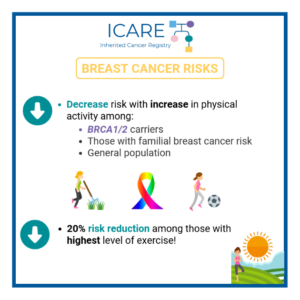
Several important studies were published recently on the effectiveness of risk management strategies in BRCA carriers. Specifically, a recently published study in which ICARE participants were included suggested that preventive bilateral mastectomy for BRCA carriers greatly reduced the risk of developing breast cancer by 80%.1 Additionally, study findings showed that after preventive mastectomy, the chance …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2024-how-well-do-cancer-risk-management-strategies-work-among-brca-carriers/
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2024-new-guidelines-released-through-asco-society-of-oncology-germline-testing-in-patients-with-breast-center/
ICARE Newsletter Spring 2024
National Comprehensive Cancer Network (NCCN) Guideline Updates
ICARE Newsletter Spring 2024
National Comprehensive Cancer Network (NCCN) Guideline Updates
Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic Cancer – Released February 12th, 2024 (V3.2024) Check out the full guidelines by creating a FREE account at www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf Contralateral breast cancer risks in these updated guidelines: Expanded guidance about gynecologic cancers in BRCA1/2 carriers: Some highlights related to HRT include: Genetic/Familial High-Risk Assessment: Colorectal Cancer – Released …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2024-national-comprehensive-cancer-network-nccn-guideline-updates/
ICARE Social Media Post March 2024
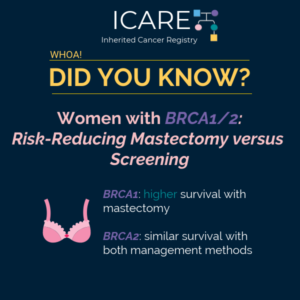
Risk Management Strategies for Women with BRCA1/2
ICARE Social Media Post March 2024
Risk Management Strategies for Women with BRCA1/2
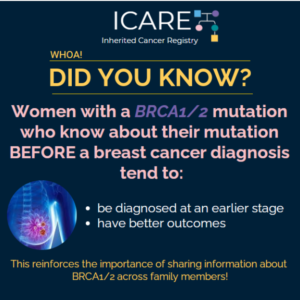
Two new research studies that 𝗶𝗻𝗰𝗹𝘂𝗱𝗲𝗱 𝗜𝗖𝗔𝗥𝗘 𝗽𝗮𝗿𝘁𝗶𝗰𝗶𝗽𝗮𝗻𝘁𝘀 showed that risk of death is lowered among BRCA1/2 carriers with 1) MRI screening for breast cancer and 2) removal of both ovaries and fallopian tubes. 💡 In the first study, MRI screening greatly lowered the risk of death from breast cancer (hazard ratio of 0.23) with …
Permanent link to this article: https://inheritedcancer.net/post3524/
ICARE Social Media Post February 2024
Risk-reducing mastectomy and breast cancer mortality in women with BRCA1/BRCA2
ICARE Social Media Post February 2024
Risk-reducing mastectomy and breast cancer mortality in women with BRCA1/BRCA2
🔬 Exciting news! A recent study, which included ICARE participants, suggests the life-saving potential of preventive mastectomy for BRCA1 and BRCA2 carriers. 💪 This procedure significantly reduces the risk of developing breast cancer and may even decrease the risk of breast cancer-related mortality. The study found that after preventive mastectomy, the chance of dying from …
Permanent link to this article: https://inheritedcancer.net/post22724/
PARP Inhibitor (Olaparib) in men with BRCA mutations and prostate cancer
A recent study found that Olaparib (Lynparza) improved survival outcomes among men with BRCA1/2 mutations and metastatic castration-resistant prostate cancer, regardless of whether the mutation was germline or somatic. This underscores the potential of targeted therapies in improving outcomes for those with inherited cancer gene mutations. Learn more at: https://ascopubs.org/doi/10.1200/JCO.23.00339 Reference: Mateo, et al. J …
Permanent link to this article: https://inheritedcancer.net/post22024/
ICARE Social Media Post February 2024
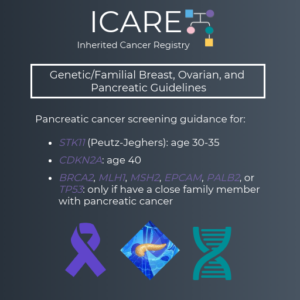
Updates to NCCN Guidelines: Genetic/Familial Breast, Ovarian, and Pancreatic Post #1
ICARE Social Media Post February 2024
Updates to NCCN Guidelines: Genetic/Familial Breast, Ovarian, and Pancreatic Post #1
The National Comprehensive Cancer Network (NCCN) just released updated Genetic/Familial Breast, Ovarian, and Pancreatic Cancer guidelines on February 12th, 2024! Updates include adding contralateral breast cancer risks for BRCA1, BRCA2, PALB2, CHEK2, and other genes to the GENE-A (Cancer Risk Management) table 🧬 You can check out the full guidelines by creating a FREE account …
Permanent link to this article: https://inheritedcancer.net/post21324/
ICARE Social Media Post February 2024 Updates to NCCN Guidelines: Genetic/Familial Breast, Ovarian, and Pancreatic Post #2
The National Comprehensive Cancer Network (NCCN) just released updated Genetic/Familial Breast, Ovarian, and Pancreatic Cancer guidelines on February 12th, 2024! Updates include expanded guidance about gynecologic cancers in BRCA1 and BRCA2, including:✓ Reproductive considerations✓ Non-surgical and surgical risk reduction✓ Salpingectomy✓ Hysterectomy considerations✓ HRT after risk-reducing removal of the ovaries You can check out the full …
Permanent link to this article: https://inheritedcancer.net/post21324_2/
Permanent link to this article: https://inheritedcancer.net/post10524/
ICARE Social Media Post January 2023
BRCA/Prostate Cancer/Treatment
ICARE Social Media Post January 2023
BRCA/Prostate Cancer/Treatment
A recent study suggests that Platinum Chemotherapy is as effective as PARP inhibitors for individuals with BRCA-positive metastatic castration-resistant prostate cancer. The study sheds light on treatment options for advanced prostate cancer patients 🩺✨ Learn more at: http://tinyurl.com/3vs2mk8f Reference: Fazekas, et al. Eur Urol Oncol. 2023: S2588-9311(23)00174-8. PMID: 37722977.
Permanent link to this article: https://inheritedcancer.net/post10324/
ICARE Social Media Post December 2023
BRCA1/2 carriers with Risk Reducing Salpingo-oophorectomy
ICARE Social Media Post December 2023
BRCA1/2 carriers with Risk Reducing Salpingo-oophorectomy
A study among BRCA1/2 carriers who underwent a risk-reducing salpingo-oophorectomy (i.e., removal of both ovaries and fallopian tubes) found that the risk of peritoneal cancer increases if serous tubal intraepithelial carcinoma (STIC) is present – specifically, peritoneal cancer risk was 10.5% with STIC versus 0.3% without STIC at 5 years and 27.5% with STIC versus …
Permanent link to this article: https://inheritedcancer.net/post121923/
ICARE Social Media Post December 2023
BRCA1/2: removing both ovaries and tubes to lower risks
ICARE Social Media Post December 2023
BRCA1/2: removing both ovaries and tubes to lower risks
A recent study among nearly 500 BRCA1/2 carriers who underwent breast cancer surgery found that survival was higher in those who chose preventive removal of their ovaries and fallopian tubes (in order to reduce cancer risks) compared to those who did not, particularly in BRCA1 carriers. Learn more at: https://tinyurl.com/5hdx6ytv Reference: Martelli, et al. JAMA …
Permanent link to this article: https://inheritedcancer.net/post121323/
ICARE Social Media Post December 2023
Tamoxifen and Breast Cancer Risk in BRCA Mutations
ICARE Social Media Post December 2023
Tamoxifen and Breast Cancer Risk in BRCA Mutations
In a recent study among female BRCA1/2 carriers, including ICARE participants, researchers explored the impact of using tamoxifen and/or raloxifene on breast cancer risk. 👩🔬 After almost 7 years of follow-up, only 10.9% of those in the tamoxifen/raloxifene group were diagnosed with breast cancer, compared to 14.3% of those in the non-user group. These findings …
Permanent link to this article: https://inheritedcancer.net/post120523/
Permanent link to this article: https://inheritedcancer.net/post110923/
ICARE Social Media Post October 2023
Triple-negative breast cancers across populations
ICARE Social Media Post October 2023
Triple-negative breast cancers across populations
Triple-negative breast cancers, which do not have estrogen, progesterone, or HER2 receptors, can be more serious and difficult to treat. Inherited breast cancer gene mutations, like BRCA1/2, are more common among this type of breast cancer – which is why it is important for those with triple-negative breast cancer to consider getting genetic testing that …
Permanent link to this article: https://inheritedcancer.net/post102223/
ICARE Social Media Post October 2023
BRCA1/2 Carriers with Risk-Reducing Salpingo-Oophorectomy: Cancer Worry?
ICARE Social Media Post October 2023
BRCA1/2 Carriers with Risk-Reducing Salpingo-Oophorectomy: Cancer Worry?
A study found that the majority of BRCA1/2 carriers who underwent risk-reducing salpingo-oophorectomy have declining cancer worry. However, a subset had concerns – these individuals are important to identify and try to offer additional support to. Use the following link to learn more: https://bit.ly/3qflyig Reference: van Bommel, et al. Support Care Cancer. 2022;30(4):3409-3418. PMID: 34997316.
Permanent link to this article: https://inheritedcancer.net/post102023/
ICARE Social Media Post October 2023
Navigating BRCA1/2 Choices
ICARE Social Media Post October 2023
Navigating BRCA1/2 Choices
A recent randomized controlled trial among 107 BRCA1/2 carriers, which included ICARE participants, evaluated the effectiveness of a behavioral phone intervention delivered by genetic counselors on the uptake of risk-reducing salpingo-oophorectomy. Women who received the intervention had significantly lower decisional conflict and higher knowledge after one year, and at the two year mark, nearly 54% …
Permanent link to this article: https://inheritedcancer.net/post101223/
ICARE Social Media Post October 2023
BRCA2 & Melanoma Risk
ICARE Social Media Post October 2023
BRCA2 & Melanoma Risk
Are BRCA2 carriers at higher risk for developing melanoma? As featured in our Fall 2023 newsletter, at present, we are not sure because results from different studies have been inconsistent. For instance, one study among 173 families with a BRCA2 mutation found a 2.6-fold risk of developing melanoma; however, another study of 139 Dutch families …
Permanent link to this article: https://inheritedcancer.net/post100623/
ICARE Newsletter Fall 2023
Community Spotlight
ICARE Newsletter Fall 2023
Community Spotlight
My paternal grandparents were my heroes. Wise beyond their time, they relished teaching our familythat knowledge is power, health is everything, and love is unconditional. Back then, Prevention healthmagazine and vitamin supplements filled their mailbox and 1960’s exercise guru Jack LaLane, and health foodadvocate Euell Gibbons, beckoned new followers from a talking picture box in …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2023-community-spotlight/
ICARE Newsletter Fall 2023
BRCA-associated Prostate Cancers
ICARE Newsletter Fall 2023
BRCA-associated Prostate Cancers
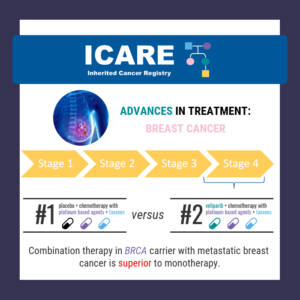
On April 28th, 2023, the FDA approved olaparib plus abiraterone acetate for first line treatment for metastatic castration-resistant prostate cancer, but only in patients whose tumors have BRCA mutations. Although a broad indication for the combination therapy was desired, concerns about the trial design were raised, and the phase III results did not explicitly show …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2023-brca-associated-prostate-cancers/
ICARE Newsletter Fall 2023
Genes Associated with Aggressive Prostate Cancer
ICARE Newsletter Fall 2023
Genes Associated with Aggressive Prostate Cancer
A new study of almost 18,000 men with prostate cancer showed that inherited mutations in the BRCA2, ATM, and NBN genes were strongly associated with aggressive prostate cancer. Less strong associations were seen for inherited mutations in the MSH2, XRCC2, and MRE11A genes. The findings of this study suggest that knowing about inherited genes that …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2023-genes-associated-with-aggressive-prostate-cancer/
ICARE Newsletter Fall 2023
Are BRCA2 Carriers at Higher Risk for Melanoma?
ICARE Newsletter Fall 2023
Are BRCA2 Carriers at Higher Risk for Melanoma?
At present, we are not sure because results from different studies have been inconsistent (1). For instance, one study among 173 families with a BRCA2 mutation found a 2.6-fold risk of developing melanoma (2); however, another study of 139 Dutch families with a BRCA2 mutation revealed a lower than general population risk for melanoma (3). …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2023-are-brca2-carriers-at-higher-risk-for-melanoma/
ICARE Newsletter Fall 2023
Fertility Treatment in BRCA Carriers: Breast Cancer Risk
ICARE Newsletter Fall 2023
Fertility Treatment in BRCA Carriers: Breast Cancer Risk
A recent study among female BRCA carriers showed risk of breast cancer was not significantly raised through fertility treatment, which is reassuring. There remains a need to further study this question in more detail, for associations with breast cancers across different breast cancer subtypes, including breast cancers that are hormone-related and breast cancers that are …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2023-fertility-treatment-in-brca-carriers-breast-cancer-risk/
ICARE Newsletter Fall 2023
Tamoxifen and Breast Cancer Risk in Women with a BRCA1 or BRCA2 Mutation
ICARE Newsletter Fall 2023
Tamoxifen and Breast Cancer Risk in Women with a BRCA1 or BRCA2 Mutation
In a study of female BRCA carriers, including ICARE participants, those that used tamoxifen and/or raloxifenewere compared to those that did not. After an average follow-up time of almost 7 years, 10.9% of those in the tamoxifen/raloxifene group were diagnosed with breast cancer, compared to 14.3% in the group that did not use thesedrugs. This …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2023-tamoxifen-and-breast-cancer-risk-in-women-with-a-brca1-or-brca2-mutation/
ICARE Newsletter Fall 2023
BRCA1 and BRCA2 Carriers: Cancer Risks with Oral Contraceptive Use (UK)
ICARE Newsletter Fall 2023
BRCA1 and BRCA2 Carriers: Cancer Risks with Oral Contraceptive Use (UK)
Among female BRCA1 and BRCA2 carriers, a recent study found that oral contraceptive use is associated with:› Raised risk of breast cancer, but only in those using for over 5 years (relative risk: 1.25)› Lower risk of ovarian cancer (nearly cut in half) Park, et al. Carcinogenesis. 2022;43(3):231-242. PMID:
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2023-brca1-and-brca2-carriers-cancer-risks-with-oral-contraceptive-use-uk/
Permanent link to this article: https://inheritedcancer.net/post100423/
Permanent link to this article: https://inheritedcancer.net/post83032/
ICARE Social Media Post August 2023
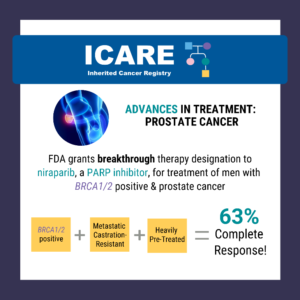
FDA Approval Post – Approves Niraparib
ICARE Social Media Post August 2023
FDA Approval Post – Approves Niraparib
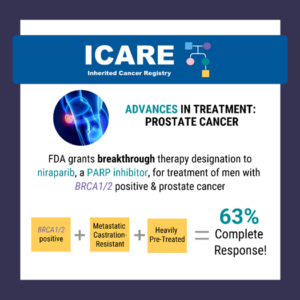
A critical step forward in cancer care! The FDA has approved the use of niraparib and abiraterone acetate with prednisone in treating patients with BRCA-mutated castration-resistant prostate cancer. The green light comes backed by the robust efficacy data from the MAGNITUDE trial. Read more about the FDA approval at https://tinyurl.com/dzdvtm9m Learn more about the MAGNITUDE …
Permanent link to this article: https://inheritedcancer.net/post82423/
ICARE Featured Video August 2023
SELECT: Using a Gene Expression-driven Algorithm to Prioritize Competing Treatment Options for BRCA Carriers with Breast Cancer
ICARE Featured Video August 2023
SELECT: Using a Gene Expression-driven Algorithm to Prioritize Competing Treatment Options for BRCA Carriers with Breast Cancer
Below is a featured video from the August 2023 case conference, during which Sheila Rajagopal, MD, MPH, MSc from the National Cancer Institute presents on using a gene expression-driven algorithm to prioritize competing treatment options for BRCA carriers with breast cancer.
Permanent link to this article: https://inheritedcancer.net/video81023/
Permanent link to this article: https://inheritedcancer.net/post81123/
ICARE Social Media Post July 2023
Inherited Cancer Genes in Children: BRCA1/2, PALB2, ATM, CHEK2 , Lynch Genes
ICARE Social Media Post July 2023
Inherited Cancer Genes in Children: BRCA1/2, PALB2, ATM, CHEK2 , Lynch Genes
Recent study findings suggest that BRCA1/2, PALB2, ATM, CHEK2, and the Lynch Syndrome genes might confer reduced penetrance cancer risk among children. However, there are no adjustments to management or testing recommendations based on the level of risk (i.e., normally do not test children for conditions that primarily increase the risk of cancer in adulthood). …
Permanent link to this article: https://inheritedcancer.net/post72123/
ICARE Social Media Post July 2023
BRCA1/2 carriers: Risks with Oral Contraceptives Use
ICARE Social Media Post July 2023
BRCA1/2 carriers: Risks with Oral Contraceptives Use
Among female BRCA1 and BRCA2 carriers, a recent study found that oral contraceptive use is associated with an increased risk of breast cancer, but only among users who have been using them for more than five years, while ovarian cancer risk was nearly cut in half. Use the link in bio to learn more! Reference: …
Permanent link to this article: https://inheritedcancer.net/post70623/
ICARE Social Media Post June 2023
FDA Advisory Committee Recommendation: Olaparib for Prostate Cancer Treatment
ICARE Social Media Post June 2023
FDA Advisory Committee Recommendation: Olaparib for Prostate Cancer Treatment
Olaparib plus abiraterone acetate is recommended as the first line treatment for metastatic castration-resistant prostate cancer, but only in patients whose tumors have BRCA mutations. Although a broad indication for the combination therapy was desired, concerns about the trial design were raised, and the phase III results did not explicitly show that patients without a …
Permanent link to this article: https://inheritedcancer.net/post61623/
ICARE Social Media Post May 2023
PARP Inhibitors for Metastatic Prostate Cancer: Talazoparib
ICARE Social Media Post May 2023
PARP Inhibitors for Metastatic Prostate Cancer: Talazoparib
A study that compared a PARP inhibitor (talazoparib) to the standard of care (an androgen receptor inhibitor) in metastatic castration-resistant prostate cancer found that it improved progression-free survival regardless of BRCA mutation status. However, the benefit was greatest in males with a BRCA or other DNA repair gene mutation. Read the full article at this …
Permanent link to this article: https://inheritedcancer.net/post50823/
ICARE Social Media Post April 2023
Cancer Risks in Older Females with a BRCA Mutation
ICARE Social Media Post April 2023
Cancer Risks in Older Females with a BRCA Mutation
A recent study among over 2200 females (aged 50-75) with BRCA mutations, including ICARE participants, found 379 diagnosed cancers with breast and ovarian cancer being the most common cancers observed. Overall cancer risks were 49% in BRCA1 carriers and 43% in BRCA2 carriers, and cancer risks dropped to 9% among those who had preventative removal …
Permanent link to this article: https://inheritedcancer.net/post42823/
Permanent link to this article: https://inheritedcancer.net/post42123/
ICARE Social Media Post April 2023
PARP Inhibitors for Metastatic Prostate Cancer: Niraparib
ICARE Social Media Post April 2023
PARP Inhibitors for Metastatic Prostate Cancer: Niraparib
A study that evaluated a PARP inhibitor (niraparib) in males with metastatic prostate cancer found that those with a BRCA1 or BRCA2 mutation lived longer on average than those who did not have a BRCA mutation. Read the full article at the link: https://pubmed.ncbi.nlm.nih.gov/35131040/Reference:Smith et al. Lancet Oncol. 2022;23(3):362-373. PMID: 35131040.
Permanent link to this article: https://inheritedcancer.net/post42023/
ICARE Newsletter Spring 2023
Prostate Cancer Treatment Updates
ICARE Newsletter Spring 2023
Prostate Cancer Treatment Updates
A study to test niraparib (a PARP inhibitor) in males with metastatic prostate cancer showed that those with an inherited BRCA1 or BRCA2 (BRCA) mutation lived longer on average compared to those without a BRCA mutation. Side effects from niraparib were similar to those previously reported with PARP inhibitors.1 Another PARP inhibitor trial tested an …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2023prostate-cancer-treatment-updates/
ICARE Newsletter Spring 2023
Breast Cancer Treatment Updates
ICARE Newsletter Spring 2023
Findings from a Phase II study to evaluate the use of talazoparib (a PARP inhibitor) in individuals with advanced PALB2-mutation breast cancer showed that it appeared effective in certain patients and appeared safe (with similar adverse events as those previously reported with this drug).1 There are several Phase II trials to evaluate PARP inhibitors in …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2023-breast-cancer-treatment-updates/
ICARE Newsletter Spring 2023
Breast Cancer Screening in Male BRCA1/2 Carriers
ICARE Newsletter Spring 2023
Generally, males with breast cancer present with advanced stage disease, thought to be due to a lack of screening. While data to determine performance of breast screening through mammograms for males at inherited risk is limited, recent studies suggest that the detection rate is similar or better than for females at general population risk.1,2,3 In …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2023-breast-cancer-screening-in-male-brca1-2-carriers/
ICARE Newsletter Spring 2023
Lynch Syndrome: Colorectal Cancer Risks Revisited
ICARE Newsletter Spring 2023
While there are higher cancer risks in BRCA mutation carriers starting in the mid-20s, a recent study focused on studying cancer risks in older females aged 50-75. Of the over 2000 females in the study, which included ICARE participants, 379 cancers were found between age 50 to 75 with risks of 49% in BRCA1 carriers …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2023-lynch-syndrome-colorectal-cancer-risks-revisited/
ICARE Newsletter Spring 2023
Inherited Breast Cancer: Contralateral Breast Cancer Risks
ICARE Newsletter Spring 2023
Inherited Breast Cancer: Contralateral Breast Cancer Risks
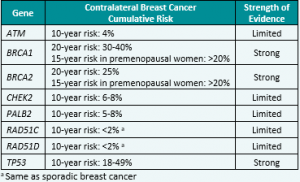
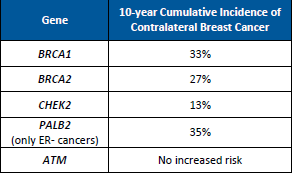
While higher risks for contralateral breast cancer (CBC) have been known for BRCA1 and BRCA2, a newly published study demonstrated that the risk of CBC is also higher for female PALB2 and CHEK2 carriers; however, no elevated risks were found for ATM carriers (Table 1).1 This information is important to study, as it may be …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2023-inherited-breast-cancer-contralateral-breast-cancer-risks/
ICARE Newsletter Spring 2023
National Comprehensive Cancer Network (NCCN) Guidelines Updates
ICARE Newsletter Spring 2023
National Comprehensive Cancer Network (NCCN) Guidelines Updates
Check out the full NCCN guidelines by creating a FREE account at www.nccn.org Genetic/Familial High-Risk Assessment: Breast, Ovarian, PancreaticJanuary 10th, 2023 (Version 2.2023) focused on male BRCA carriers:› Consider annual mammograms (particularly in BRCA2 carriers) starting at age 50 or 10 years before the earliest male breast cancer in the family (whichever comes first)February 13th, …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2023-national-comprehensive-cancer-network-nccn-guidelines-updates/
ICARE Social Media Post March 2023
BGREAT December 2022 Newsletter
ICARE Social Media Post March 2023
BGREAT December 2022 Newsletter
Check out the latest edition of our B-GREAT newsletter for updates about inherited cancers in the context of racial inequalities in healthcare. You can read the newsletter by visiting 👇https://bgreatinitiative.inheritedcancer.net/wp-content/uploads/BGREAT-December-2022-Newsletter.pdf Please feel free to share with family members, friends, and/or your healthcare providers.
Permanent link to this article: https://inheritedcancer.net/post30623/
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2022-ask-the-expert/
ICARE Newsletter Spring 2022
Inherited Cancer Treatment Updates
ICARE Newsletter Spring 2022
Inherited Cancer Treatment Updates
Lynch Syndrome Carriers with Advanced Uterine Cancer: Treatment with PembrolizumabWomen with Lynch Syndrome are at high risk for uterine cancer. The type of uterine cancer they develop has the tumorcharacteristic of being ‘MSI-H’. A new study indicated treatment with pembrolizumab (Keytruda) resulted in benefit inpatients with MSI-H advanced uterine cancer. Von Hippel-Lindau Patients: Treatment of …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2022-inherited-cancer-treatment-updates/
ICARE Newsletter Spring 2022
BRCA1/2 Oral Contraceptives and Breast Cancer
ICARE Newsletter Spring 2022
BRCA1/2 Oral Contraceptives and Breast Cancer
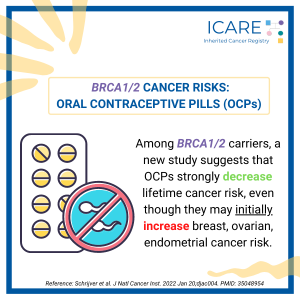
A new study found that among BRCA1/2 carriers, oral contraceptive use strongly lowered cancer risk over one’s lifetime, even though at first, they raise risks of breast, ovarian, and endometrial cancer.Schrijver et al. J Natl Cancer Inst. 2022 Jan. PMID: 35048954. Social media post February 15th, 2022. Available at:https://tinyurl.com/post21522.
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2022-brca1-2-oral-contraceptives-and-breast-cancer/
ICARE Newsletter Spring 2022
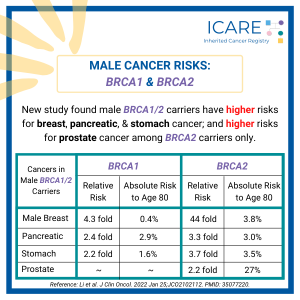
BRCA1/2 and Male Cancer Risks
ICARE Newsletter Spring 2022
BRCA1/2 and Male Cancer Risks
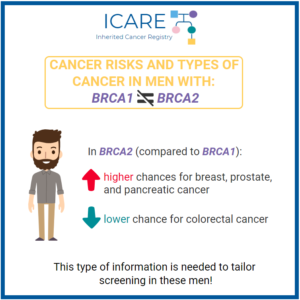
A recent international study found that male BRCA1 and BRCA2 carriers have a higher risk for breast, pancreatic, and stomach cancer. Additionally, male BRCA2 carriers were found to have higher risks for prostate cancer. See the below table for the specific risk levels: Li et al. J Clin Oncol. 2022 Jan. PMID: 35077220. Social media …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2022-brca1-2-and-male-cancer-risks/
ICARE Newsletter Spring 2022
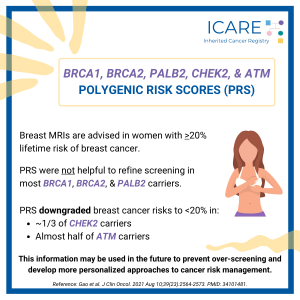
Polygenic Risk Scores and Inherited Breast Cancer Genes: BRCA1/2, PALB2, CHEK2, and ATM
ICARE Newsletter Spring 2022
Polygenic Risk Scores and Inherited Breast Cancer Genes: BRCA1/2, PALB2, CHEK2, and ATM
Breast MRIs are advised in women with >20% lifetime risk of breast cancer. A new study showed that breast cancer risks in BRCA1, BRCA2 and PALB2 carriers remained higher than 20%, regardless of whether polygenic risk scores (PRS) were done, suggesting this is of limited help in refining screening. In contrast, PRS downgraded breast cancer …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2022-polygenic-risk-scores-and-inherited-breast-cancer-genes-brca1-2-palb2-chek2-and-atm/
ICARE Newsletter Fall 2022
Community Spotlight
ICARE Newsletter Fall 2022
Community Spotlight
When I was just 8 years old my mother was diagnosed with a very aggressive breast cancer. I didn’t reallyunderstand the concept of cancer at that age, but I knew what was happening was terrible. After manysurgeries and treatments, she passed away 2 years later at the age of 35. There was no hereditary cancertesting …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2022-community-spotlight/
ICARE Newsletter Fall 2022
Ask the Expert
ICARE Newsletter Fall 2022
Ask the Expert
The below question was addressed by Dr. Kamran Idrees, Chief of the Division of Surgical Oncology & Endocrine Surgery, Associate Professor of Surgery, Ingram Associate Professor of Cancer Research, and Director of Pancreatic and Gastro Intestinal Surgical Oncology at Vanderbilt-Ingram Cancer Center. Dr. Idrees’ research has focused on colorectal cancer, liver metastases, and pancreatic cancer …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2022-ask-the-expert/
ICARE Newsletter Fall 2022
Screening & Treatment Updates: Pancreatic Cancer
ICARE Newsletter Fall 2022
Screening & Treatment Updates: Pancreatic Cancer
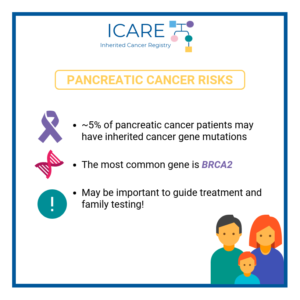
A recent small study suggests that immunotherapy may benefit patients with refractory pancreatic or biliary cancer who have inherited a mutation in the homologous recombination deficiency (HRD) genes, BRCA1, BRCA2, and RAD51C.Another new study reported that in BRCA1/2 carriers with pancreatic cancer, maintenance treatment with Olaparib may be of benefit. Findings showed that with Olaparib, …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2022-screening-treatment-updates-pancreatic-cancer/
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2022-which-genes-are-confirmed-as-inherited-breast-cancer-genes/
ICARE Newsletter Fall 2022
Inherited Cancer Genes: New Associations
ICARE Newsletter Fall 2022
Inherited Cancer Genes: New Associations
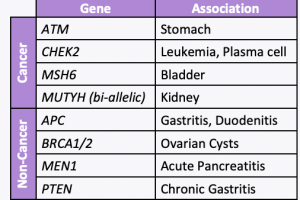
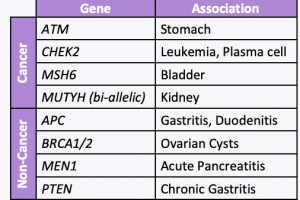
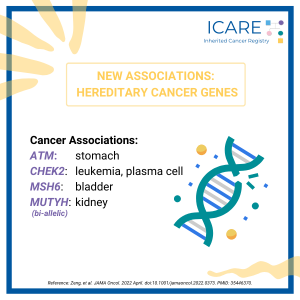
A new study led by colleagues at Vanderbilt University Medical Center, including our clinical geneticist colleague, Dr. Georgia Wiesner, evaluated 23 hereditary cancer genes and found 19 new gene associations including 7 new associations with cancer and 12 new associations with noncancer diseases. The associations with cancer versus other conditions is included in the table. …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2022-inherited-cancer-genes-new-associations/
ICARE Newsletter Fall 2022
BRCA1/2 Cancer Risk Updates
ICARE Newsletter Fall 2022
BRCA1/2 Cancer Risk Updates
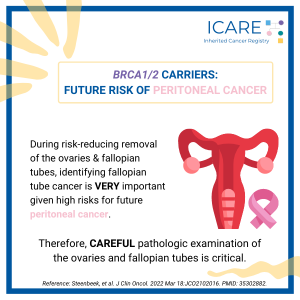
During preventive surgery to remove the ovaries and fallopian tubes (called a risk-reducing salpingo-oophorectomy orRRSO), a new study found that the detection of tubal intraepithelial carcinoma predicts the risk of later peritonealcancer.1 These findings show the importance of timely RRSO and the need to do a careful pathology exam of the ovaries and fallopian tubes …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2022-brca1-2-cancer-risk-updates/
ICARE Newsletter Fall 2022
National Comprehensive Cancer Network (NCCN) Guidelines Updates
ICARE Newsletter Fall 2022
National Comprehensive Cancer Network (NCCN) Guidelines Updates
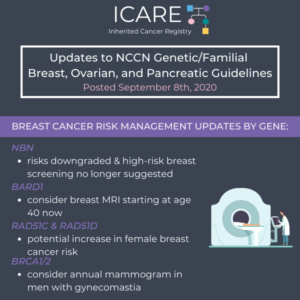
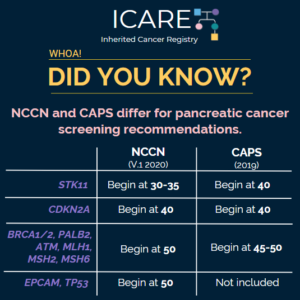
Check out the full NCCN guidelines by creating a FREE account at www.nccn.org Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic – Released September 7th, 2022› Testing eligibility based on personal history of any type of breast cancer in females was updated from age ≤45 to ≤50 making more females with breast cancer eligible for testing …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2022-national-comprehensive-cancer-network-nccn-guidelines-updates/
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2021-ask-the-expert/
ICARE Newsletter Fall 2021
>
Polygenic Risk Scores and Breast Cancer Risks: BRCA1/2, PALB2, CHEK2, ATM , and beyond!
ICARE Newsletter Fall 2021
>Polygenic Risk Scores and Breast Cancer Risks: BRCA1/2, PALB2, CHEK2, ATM , and beyond!
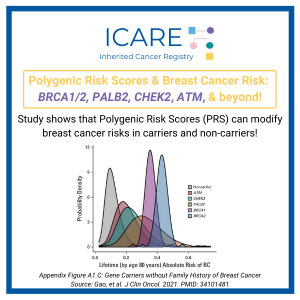
A recent study found use of a polygenic risk score (PRS) modified the estimated riskof breast cancer among both carriers and non-carriers of inherited breast cancerpredisposition genes. Taking PRS into account, more than 95% of BRCA1, BRCA2,and PALB2 carriers had greater than 20% lifetime risks of breast cancer. In contrast,among ATM and CHEK2 carriers without …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2021-polygenic-risk-scores-and-breast-cancer-risks-brca1-2-palb2-chek2-atm-and-beyond/
Permanent link to this article: https://inheritedcancer.net/post40123/
Newsletter Fall 2021
Inherited Cancer Treatment Updates
Newsletter Fall 2021
Inherited Cancer Treatment Updates
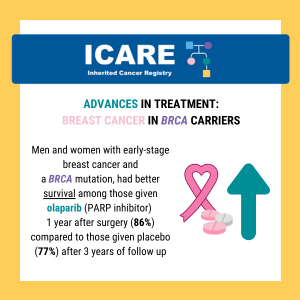
Early-stage, high-risk breast cancer in BRCA carriers: Results of the highly awaited phase 3 OlympiA trial showed promising results for EARLY STAGE (i.e., localized Stage 2-3) high-risk breast cancer patients with a BRCA mutation who were treated with a PARP inhibitor (olaparib) in the adjuvant setting (i.e., AFTER surgery).1 Early-stage breast cancer in this trial …
Permanent link to this article: https://inheritedcancer.net/newsletter-fall-2021-inherited-cancer-treatment-updates/
Newsletter Fall 2021
Modifying Risks in BRCA Carriers
Newsletter Fall 2021
Modifying Risks in BRCA Carriers
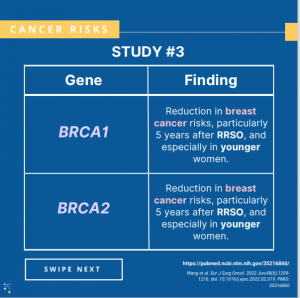
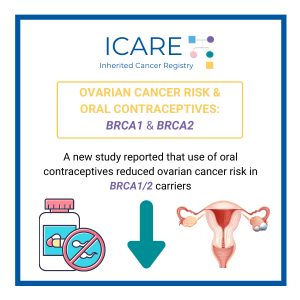
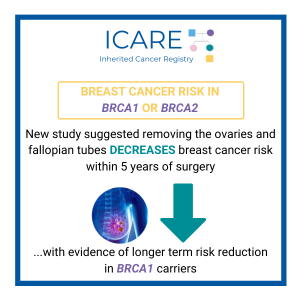
Breast cancer risks: A risk-reducing salpingo-oophorectomy (i.e., removal of both ovaries and fallopian tubes) in BRCA carriers was associated with a reduced risk of breast cancer within five years after surgery, with evidence of longer-term risk reduction among those with BRCA1 variants.1 Ovarian cancer risks: A new study reported that the use of oral contraceptives …
Permanent link to this article: https://inheritedcancer.net/newsletter-fall-2021-modifying-risks-in-brca-carriers/
Newsletter Fall 2021
Breast Cancer Risks Remain High in PALB2 & BRCA
Newsletter Fall 2021
Breast Cancer Risks Remain High in PALB2 & BRCA
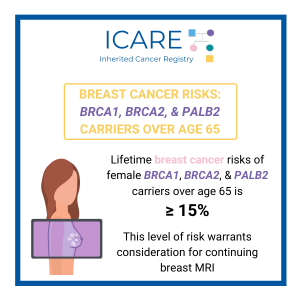
A new study found that lifetime breast cancer risk is 15% or more in female BRCA1, BRCA2, and PALB2 carriers over age 65. This level of risk warrants consideration for continuing breast MRI.1 These results are similar to those of a study that included ICARE participants,2 which reported the risk of developing breast cancer remains …
Permanent link to this article: https://inheritedcancer.net/newsletter-fall-2021-breast-cancer-risks-remain-high-in-palb2-brca/
Newsletter Fall 2021
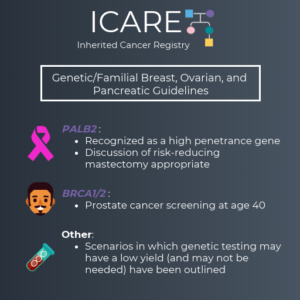
PALB2 : Increasingly Recognized as the Third Most Important Inherited Breast Cancer Gene
Newsletter Fall 2021
PALB2 : Increasingly Recognized as the Third Most Important Inherited Breast Cancer Gene
In May 2021, a clinical practice resource was released by the American College of Medical Genetics and Genomics (ACMG) from a global team of cancer genetics specialists (see figure) to help guide the care of PALB2 carriers.1 PALB2 is considered the third most important breast cancer risk gene, after BRCA1 and BRCA2, with PALB2 carriers …
Permanent link to this article: https://inheritedcancer.net/div-classboxedspan-stylecolor-whiteh6newsletter-fall-2021-h6-span-divbrcenterh4span-stylecolor-56b0e4-ipalb2-i-increas/
Newsletter Fall 2021
Updates to NCCN Genetic/Familial High-Risk Assessment
Newsletter Fall 2021
Updates to NCCN Genetic/Familial High-Risk Assessment
Breast, Ovarian, and Pancreatic Guidelines V.1.2022: Released August 11th, 2021 Colorectal Cancer Guidelines V.1.2021: Released May 11th, 2021 Check out the full NCCN guidelines by creating a FREE account at www.nccn.org
Permanent link to this article: https://inheritedcancer.net/newsletter-fall-2021-updates-to-nccn-genetic-familial-high-risk-assessment/
ICARE Social Media Post January 2023
Inherited Breast Cancers Across Populations
ICARE Social Media Post January 2023
Inherited Breast Cancers Across Populations
Did you know that BRCA1/2 are amongst the most well-studied genes, yet most BRCA1/2 studies have been done in White populations? This means our knowledge about genes and risks comes primarily from White populations. • Some research suggests that BRCA1/2 gene mutations may be more common in young Black women with breast cancer. • Even …
Permanent link to this article: https://inheritedcancer.net/post12423-2/
ICARE Social Media Post January 2023
Updates to NCCN Guidelines: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic
ICARE Social Media Post January 2023
Updates to NCCN Guidelines: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic
The National Comprehensive Cancer Network (NCCN) just released updated breast, ovarian, and pancreatic cancer guidelines which included updated breast cancer screening recommendations for male BRCA carriers (particularly male BRCA2 carriers). It is now recommended that male BRCA carriers consider annual mammograms starting at age 50 or 10 years before the earliest male breast cancer in …
Permanent link to this article: https://inheritedcancer.net/post12423/
ICARE Social Media Post January 2023
New Contralateral Breast Cancer Risk Prediction Model for BRCA1/2 Carriers
ICARE Social Media Post January 2023
New Contralateral Breast Cancer Risk Prediction Model for BRCA1/2 Carriers
A new risk prediction model was developed to assess the risk of contralateral breast cancer in BRCA1/2 carriers. Risks are higher with:• Younger age at first breast cancer• Close family member with breast and/or ovarian cancer• Mutation located near the 3′ region of the gene Risks are lower with:• Endocrine therapy Use this link in …
Permanent link to this article: https://inheritedcancer.net/post11423/
ICARE Social Media Post December 2022
Bilateral Mastectomy in BRCA1/2, PALB2, ATM, & CHEK2 Carriers
ICARE Social Media Post December 2022
Bilateral Mastectomy in BRCA1/2, PALB2, ATM, & CHEK2 Carriers
A recent study including data from ICARE participants found similar rates of bilateral mastectomy across high (BRCA1, BRCA2, PALB2) and moderate (ATM, CHEK2) penetrance genes. The high rates of bilateral mastectomies seen in those with moderate penetrance genes is concerning for overtreatment. Use the link to learn more: https://jamanetwork.com/journals/jamaoncology/fullarticle/2797978?guestAccessKey=fe9a3a20-8623-4feb-a0c5-315ad43a8fcb&utm_source=jps&utm_medium=email&utm_campaign=author_alert-jamanetwork&utm_content=author-author_engagement&utm_term=1m Reference: Reid et al. Receipt of …
Permanent link to this article: https://inheritedcancer.net/post120822/
ICARE Social Media Post October 2022
Ovarian Cancer Risks: Weight Gain in BRCA1/2 Carriers
ICARE Social Media Post October 2022
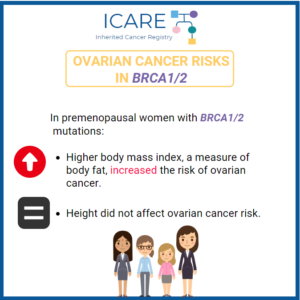
Ovarian Cancer Risks: Weight Gain in BRCA1/2 Carriers
A study found that adult weight gain is a risk factor for ovarian cancer. This highlights the importance for BRCA1/2 carriers to maintain a healthy weight throughout adulthood.Read the full article to learn more! https://pubmed.ncbi.nlm.nih.gov/34426412/Reference: Kim et al. Cancer Epidemiol Biomarkers Prev. 2021 Nov;30(11):2038-2043. PMID: 34426412.
Permanent link to this article: https://inheritedcancer.net/post100122/
ICARE Social Media Post September 2022
Breast cancer risks: BRCA1/2 carriers with risk reducing salpingo-oophorectomy (RRSO)
ICARE Social Media Post September 2022
Breast cancer risks: BRCA1/2 carriers with risk reducing salpingo-oophorectomy (RRSO)
Three recent studies found varying results when evaluating breast cancer risk in BRCA1/2 carriers with risk-reducing salpingo-oophorectomy (RRSO). Use these links to learn more: https://pubmed.ncbi.nlm.nih.gov/31948486/ https://pubmed.ncbi.nlm.nih.gov/33630024/ https://pubmed.ncbi.nlm.nih.gov/35216860/
Permanent link to this article: https://inheritedcancer.net/post92722/
ICARE Social Media Post September 2022
Hormonal Replacement Therapy (HRT) in BRCA1 and BRCA2 carriers
ICARE Social Media Post September 2022
Hormonal Replacement Therapy (HRT) in BRCA1 and BRCA2 carriers
A recent study showed hormone replacement therapy (HRT) is reasonable to offer to BRCA1 and BRCA2 carriers who underwent risk-reducing salpingo-oophorectomy (RSSO). Read the full article at this link: https://pubmed.ncbi.nlm.nih.gov/32143914/Reference: Mills, et al. Gynecol Oncol; 2020 Jun;157(3):706-710. PMID: 32143914.
Permanent link to this article: https://inheritedcancer.net/post2922/
Permanent link to this article: https://inheritedcancer.net/post92122/
ICARE Social Media Post August 2022
Nipple sparing mastectomy: BRCA1/2 carriers
ICARE Social Media Post August 2022
Nipple sparing mastectomy: BRCA1/2 carriers
According to a recent study, a bilateral prophylactic nipple-sparing mastectomy appears to be at least equally as safe as other types of mastectomy for preventing breast cancer. Read the full article at the link: https://pubmed.ncbi.nlm.nih.gov/34342702/Reference: Stanek et al. Aesthetic Plast Surg. 2022 Apr;46(2):706-711. PMID: 34342702.
Permanent link to this article: https://inheritedcancer.net/post82922/
ICARE Social Media Post August 2022
Pancreatic Cancer Treatment
ICARE Social Media Post August 2022
Pancreatic Cancer Treatment
A new study reports that maintenance treatment with Olaparib may benefit BRCA1/2 carriers with pancreatic cancer. These findings demonstrated:long-term survival was more commontime to subsequent therapy was prolongedRead the full article at the link: https://ascopubs.org/doi/pdf/10.1200/JCO.21.01604Reference: Kindler et al. J Clin Oncol. 2022; JCO2101604. PMID: 35834777.
Permanent link to this article: https://inheritedcancer.net/post80522/
ICARE Social Media Post July 2022
Pancreatic Cancer Screening
ICARE Social Media Post July 2022
Pancreatic Cancer Screening
A recent study found that earlier diagnosis improved survival in people at high risk of pancreatic cancer.High risk was defined based on:family history and/orinherited gene mutation (BRCA1, BRCA2, CDKN2A, Lynch Syndrome genes, PALB2, ATM, and STK11)Read the article at the link: https://ascopost.com/news/june-2022/outcomes-of-pancreas-surveillance-in-the-caps5-study-and-total-caps-cohort/Reference: Dbouk, et al. J Clin Oncol. 2022 Jun 15:JCO2200298. doi: 10.1200/JCO.22.00298. PMID: 35704792.
Permanent link to this article: https://inheritedcancer.net/post72622/
ICARE Social Media Post June 2022
Refractory Pancreatic or Biliary Cancer
ICARE Social Media Post June 2022
Refractory Pancreatic or Biliary Cancer
A recent small study suggests that immunotherapy may be beneficial for patients with refractory pancreatic or biliary cancer who have inherited homologous recombination deficiency (HRD) genes, BRCA1, BRCA2, and RAD51C.Check out the full article to learn more at 👇https://jamanetwork.com/journals/jamaoncology/article-abstract/2791557Reference: Terrero et al. JAMA Oncol. 2022 Apr:e220611. doi:10.1001/jamaoncol.2022.0611. PMID: 35446342.
Permanent link to this article: https://inheritedcancer.net/post61322/
ICARE Social Media Post June 2022
New Variants Linked to Hereditary Cancer
ICARE Social Media Post June 2022
New Variants Linked to Hereditary Cancer
A new study evaluated 23 hereditary cancer genes and found 19 new gene associations, including 7 new associations with cancer and 12 new associations with non-neoplastic diseases. Specifically, the below genes were found to have an increased risk of disease:APC: benign liver/bile duct tumors, gastritis, and duodenitisATM: stomach cancer and pancreatic cancerBRCA1/2: ovarian cystsCHEK2: leukemia …
Permanent link to this article: https://inheritedcancer.net/post60122/
ICARE Social Media Post May 2022
BRCA Carriers with Risk-Reducing Salpingo-oophorectomy: Risk of Peritoneal Carcinomatosis
ICARE Social Media Post May 2022
BRCA Carriers with Risk-Reducing Salpingo-oophorectomy: Risk of Peritoneal Carcinomatosis
A new study found that among BRCA1/2 carriers, the presence of tubal intraepithelial carcinoma during risk-reducing salpingo-oophorectomy (i.e., preventive surgery to remove the ovaries and fallopian tubes) predicts the risk of later peritoneal cancer. These findings demonstrate:the importance of timely risk-reducing removal of the ovaries and fallopian tubesthat it is VERY important to have a …
Permanent link to this article: https://inheritedcancer.net/post52422/
ICARE Social Media Post May 2022
Breast Cancer Genes in Women of African Ancestry
ICARE Social Media Post May 2022
Breast Cancer Genes in Women of African Ancestry
A recent study in women of African ancestry confirmed genes previously identified to have associations with breast cancer risk (BRCA1, BRCA2, PALB2, ATM, TP53, NF1, and CHEK2) and provided new evidence of breast cancer risk for RAD51C and RAD51D, which was identified previously in European ancestry populations.Check out the full article at 👇https://pubmed.ncbi.nlm.nih.gov/35396981/Reference: Díaz-Zabala, et …
Permanent link to this article: https://inheritedcancer.net/post51722/
ICARE Social Media Post April 2022
Spring 2022 Ask the Expert
ICARE Social Media Post April 2022
Spring 2022 Ask the Expert
In every ICARE newsletter we give our participants the opportunity to have a question addressed by an expert in the field. In the latest edition, ICARE Founder, Dr. Tuya Pal, and her oncology colleague, Dr. Sonya Reid, discuss the types of inherited breast cancer and whether having an inherited form of breast cancer affects survival.Check …
Permanent link to this article: https://inheritedcancer.net/post42922/
ICARE Social Media Post April 2022
BRCA1/2 Carriers with Breast Cancer: Olaparib & Survival
ICARE Social Media Post April 2022
BRCA1/2 Carriers with Breast Cancer: Olaparib & Survival
A new study found that adjuvant olaparib significantly extended survival in BRCA1/2 carriers with HER2-negative high-risk early-stage breast cancer. Learn more at the following link: https://www.healio.com/news/hematology-oncology/20220323/adjuvant-olaparib-prolongs-survival-for-certain-patients-with-early-breast-cancer
Permanent link to this article: https://inheritedcancer.net/post41922/
ICARE Social Media Post March 2022
BRCA1/2: Asian Breast Cancer Patients
ICARE Social Media Post March 2022
BRCA1/2: Asian Breast Cancer Patients
A new study highlights the importance of customizing mutation carrier prediction models in order to improve the accuracy of predicting the likelihood of carrying a BRCA mutation in Asian breast cancer patients.Read the article for more info!https://ascopubs.org/doi/abs/10.1200/JCO.21.01647?cid=DM9826&bid=143994923Reference: Hong Ang et al. J Clin Oncol. 2022 Feb 10;JCO2101647. PMID: 35143328.
Permanent link to this article: https://inheritedcancer.net/post32522/
ICARE Social Media Post March 2022
FDA Approves Olaparib for Adjuvant Treatment of High-risk Early Breast Cancer
ICARE Social Media Post March 2022
FDA Approves Olaparib for Adjuvant Treatment of High-risk Early Breast Cancer
On March 11th, the Food and Drug Administration (FDA) approved olaparib (Lynparza) for the adjuvant treatment of BRCA1/2 carriers with human epidermal growth factor receptor 2 (HER2)-negative, high-risk early breast cancer who have been treated with neoadjuvant or adjuvant chemotherapy.Learn more at 👇https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-olaparib-adjuvant-treatment-high-risk-early-breast-cancer
Permanent link to this article: https://inheritedcancer.net/post31522/
ICARE Social Media Post February 2022
Use of Germline BRCA Testing in Patients With Ovarian Cancer and Commercial Insurance
ICARE Social Media Post February 2022
Use of Germline BRCA Testing in Patients With Ovarian Cancer and Commercial Insurance
A recent study found that only about 33% of women with ovarian cancer undergo germline BRCA1/2 testing, despite universal recommendations for such patients to have germline genetic testing. Check out the article for more information!https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2787937?resultClick=3Reference: Cham et al. JAMA Netw Open. 2022 Jan 4;5(1):e2142703. PMID: 35015069.
Permanent link to this article: https://inheritedcancer.net/post22122/
ICARE Social Media Post Month Year
Genetic Testing & Mortality Among Women with Breast or Ovarian Cancer
ICARE Social Media Post Month Year
Genetic Testing & Mortality Among Women with Breast or Ovarian Cancer
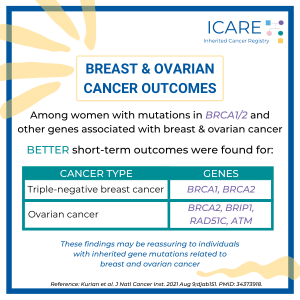
A recent study found there were BETTER short-term outcomes among women with:• triple-negative breast cancer and BRCA1 or BRCA2 mutations• ovarian cancer and BRCA2, BRIP1, RAD51C, or ATM mutationsThese findings may be reassuring to individuals with inherited gene mutations related to breast and ovarian cancer. Read the article to learn more!https://pubmed.ncbi.nlm.nih.gov/34373918/Reference: Kurian et al. J …
Permanent link to this article: https://inheritedcancer.net/post21822/
ICARE Social Media Post February 2022
BRCA1/2 Cancer Risks: Oral Contraceptives
ICARE Social Media Post February 2022
BRCA1/2 Cancer Risks: Oral Contraceptives
A new study found that among BRCA1/2 carriers oral contraceptive use strongly decreases lifetime cancer risk, despite an 𝗶𝗻𝗶𝘁𝗶𝗮𝗹 increase in breast, ovarian, and endometrial cancer risk. Read the full article to learn more!https://pubmed.ncbi.nlm.nih.gov/35048954/Reference: Schrijver et al. J Natl Cancer Inst. 2022 Jan 20;djac004. PMID: 35048954
Permanent link to this article: https://inheritedcancer.net/post21522/
Permanent link to this article: https://inheritedcancer.net/post20822/
Permanent link to this article: https://inheritedcancer.net/post20122/
ICARE Social Media Post January 2022
Male Cancer Risks: BRCA1 & BRCA2
ICARE Social Media Post January 2022
Male Cancer Risks: BRCA1 & BRCA2
A new study found that male BRCA1/2 carriers have a higher risk for breast, pancreatic, and stomach cancer. Additionally, male BRCA2 carriers were found to have higher risks for prostate cancer. Read the full Journal of Clinical Oncology article to learn more!https://ascopubs.org/doi/full/10.1200/JCO.21.02112Reference: Li et al. J Clin Oncol. 2022 Jan 25;JCO2102112. PMID: 35077220.
Permanent link to this article: https://inheritedcancer.net/post12822/
ICARE Social Media Post January 2022
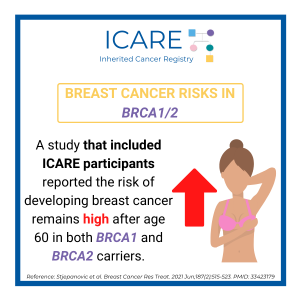
BRCA Breast Cancer Risks After Age 60
ICARE Social Media Post January 2022
BRCA Breast Cancer Risks After Age 60
A study that included ICARE participants reported the risk of developing breast cancer remains high after age 60 in both BRCA1 and BRCA2 carriers. Read the article to learn more!https://pubmed.ncbi.nlm.nih.gov/33423179/Reference: Stjepanovic et al. Breast Cancer Res Treat. 2021 Jun;187(2):515-523. PMID: 33423179
Permanent link to this article: https://inheritedcancer.net/post12522/
Permanent link to this article: https://inheritedcancer.net/post10422/
ICARE Social Media Post December 2021
Fall 2021 Ask the Expert
ICARE Social Media Post December 2021
Fall 2021 Ask the Expert
In every ICARE newsletter we give our participants the opportunity to have a question addressed by an expert in the field. In the latest edition, Dr. Kerry Schaffer discusses the use of PARP inhibitors to treat inherited forms of prostate cancer.Check out Dr. Schaffer’s full response at 👇https://inheritedcancer.net/newsletters/
Permanent link to this article: https://inheritedcancer.net/post122821/
ICARE Social Media Post December 2021
FDA Approves PARP Inhibitor (Olaparib) Treatment for some BRCA carriers with Early Stage Breast Cancer
ICARE Social Media Post December 2021
FDA Approves PARP Inhibitor (Olaparib) Treatment for some BRCA carriers with Early Stage Breast Cancer
FDA granted priority review to Olaparib for adjuvant treatment of certain patients with high-risk breast cancer. This designation applies to use of the agent by patients with BRCA-mutated, HER2-negative, high-risk early breast cancer who receive chemotherapy before or after surgery. For additional information, visit: https://tinyurl.com/healioFDAapproval
Permanent link to this article: https://inheritedcancer.net/post120621/
ICARE Social Media Post September 2021
USA Today Article: Fighting Cancer with Your Own Family History
ICARE Social Media Post September 2021
USA Today Article: Fighting Cancer with Your Own Family History
Check out the full 𝘜𝘚𝘈 𝘛𝘰𝘥𝘢𝘺 article, featuring commentary from Dr. Tuya Pal (ICARE Founder), highlighting the importance of PALB2 as an inherited breast cancer gene: https://www.futureofpersonalhealth.com/breast-health/fighting-cancer-with-your-own-family-history/# Additional guidance is available through an impactful PALB2 practice resource recently published through ACMG: https://www.acmg.net/PDFLibrary/41436_2021_1151_OnlinePDF.pdf Reference: Tischkowitz, et al. Genet Med. 2021 Aug;23(8):1416-1423. PMID: 33976419
Permanent link to this article: https://inheritedcancer.net/post92221/
Permanent link to this article: https://inheritedcancer.net/post82421/
Permanent link to this article: https://inheritedcancer.net/post82021/
ICARE Social Media Post August 2021
New York Times PALB2 Article
ICARE Social Media Post August 2021
New York Times PALB2 Article
A New York Times article just published focused on the importance of PALB2 as a breast cancer gene (https://www.nytimes.com/…/breast-cancer-palb2-brca.html), which referenced our recent article focused on managing PALB2 carriers sponsored by ACMG – American College of Medical Genetics and Genomics, and developed through a worldwide collaboration (including: Marc Tischkowicz, Judith Balmana, Will Foulkes, Paul James, …
Permanent link to this article: https://inheritedcancer.net/post81921/
Permanent link to this article: https://inheritedcancer.net/post81321/
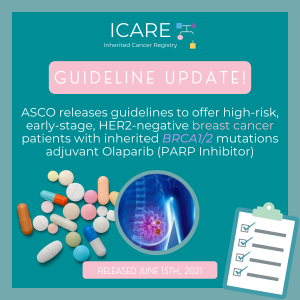
ICARE Social Media Post August 2021
ASCO Guideline Update: Olaparib for Breast Cancer
ICARE Social Media Post August 2021
ASCO Guideline Update: Olaparib for Breast Cancer
For additional information, read the updated American Society of Clinical Oncology (ASCO) recommendation (released June 15th, 2021) at the following link: https://www.asco.org/practice-patients/guidelines/breast-cancer?intcmp=ws_ascoorg_gdlns_hereditarybreastcancer_site_pressrelease_061621____#/143725
Permanent link to this article: https://inheritedcancer.net/post81021/
Permanent link to this article: https://inheritedcancer.net/post80621/
Permanent link to this article: https://inheritedcancer.net/post80321/
Permanent link to this article: https://inheritedcancer.net/post71621/
ICARE Social Media Post July 2021
Advances in Pancreatic Cancer Treatment: PALB2 & BRCA1/2
ICARE Social Media Post July 2021
Advances in Pancreatic Cancer Treatment: PALB2 & BRCA1/2
For more information, view the article at the following link below: https://ascopubs.org/doi/abs/10.1200/JCO.21.00003 You may also read the ASCO post article at: https://ascopost.com/news/may-2021/maintenance-rucaparib-in-patients-with-platinum-sensitive-pancreatic-cancer-and-germline-or-somatic-brca1-brca2-or-palb2-variants/?utm_source=TAP%2DEN%2D051221%2DTrending%5FLymphoma&utm_medium=email&utm_term=49cf1c97d48c2cf8231827e3bcb15769
Permanent link to this article: https://inheritedcancer.net/post70621/
Permanent link to this article: https://inheritedcancer.net/post62921/
Permanent link to this article: https://inheritedcancer.net/post62521/
Permanent link to this article: https://inheritedcancer.net/post62221/
Permanent link to this article: https://inheritedcancer.net/post61121/
Permanent link to this article: https://inheritedcancer.net/post50321/
Permanent link to this article: https://inheritedcancer.net/post42821/
Permanent link to this article: https://inheritedcancer.net/post40221/
Permanent link to this article: https://inheritedcancer.net/post30921/
Permanent link to this article: https://inheritedcancer.net/post30221/
Permanent link to this article: https://inheritedcancer.net/post22321/
Permanent link to this article: https://inheritedcancer.net/2nlw2021/
Permanent link to this article: https://inheritedcancer.net/1nlw2021/
ICARE Newsletter Winter 2021
Study Suggests Low Yield of MRI Surveillance After Bilateral Mastectomy
ICARE Newsletter Winter 2021
Study Suggests Low Yield of MRI Surveillance After Bilateral Mastectomy
A study of 159 women, including BRCA1/2 carriers, who had a bilateral mastectomy with reconstruction and underwent breast MRI screening, showed few women had detection of breast cancer through MRI after their bilateral mastectomy. These results support the recommendation that BRCA1/2 carriers with or without breast cancer who have a bilateral mastectomy with reconstruction do …
Permanent link to this article: https://inheritedcancer.net/8nlw2021/
ICARE Newsletter Winter 2021
Assessing How Pregnancy and Breastfeeding May Affect Cancer Risks in BRCA Carriers
ICARE Newsletter Winter 2021
Assessing How Pregnancy and Breastfeeding May Affect Cancer Risks in BRCA Carriers
Results of a recently published study suggested that pregnancy after breast cancer in BRCA carriers does not lead to a worse outcome in women or their fetuses.1 This information is reassuring for BRCA carriers who have had a prior diagnosis of breast cancer and are considering having children. In another study among female BRCA carriers …
Permanent link to this article: https://inheritedcancer.net/3nlw2021/
ICARE Newsletter Winter 2021
Inherited Cancer Treatment: Updates and Relevant Policies
ICARE Newsletter Winter 2021
Inherited Cancer Treatment: Updates and Relevant Policies
Over the last several months, the American Society of Clinical Oncology published a number of guidelines related to the use of PARP inhibitors among those with BRCA-associated cancers, including guidelines focused on ovarian cancer,1 metastatic pancreatic cancer,2 and breast cancer.3 Additionally, costs of drugs also have great potential to influence policy, highlighting the importance of …
Permanent link to this article: https://inheritedcancer.net/7nlw2021/
ICARE Social Media Post February 2021
Sharing Genetic Test Results with Family Members of BRCA, PALB2, CHEK2, and ATM Carriers
ICARE Social Media Post February 2021
Sharing Genetic Test Results with Family Members of BRCA, PALB2, CHEK2, and ATM Carriers
Our team recently published “Sharing Genetic Test Results with Family Members of 𝘉𝘙𝘊𝘈, 𝘗𝘈𝘓𝘉2, 𝘊𝘏𝘌𝘒2, and 𝘈𝘛𝘔 Carriers” in 𝘗𝘢𝘵𝘪𝘦𝘯𝘵 𝘌𝘥𝘶𝘤𝘢𝘵𝘪𝘰𝘯 𝘢𝘯𝘥 𝘊𝘰𝘶𝘯𝘴𝘦𝘭𝘪𝘯𝘨 Special Issue on Genetics. View the article available at:https://www.sciencedirect.com/science/article/pii/S0738399120306832 Challenges and barriers to family sharing included concern for family members’ reactions, complexities of information, lack of closeness, perceived relevance, & emotional impact. …
Permanent link to this article: https://inheritedcancer.net/post20821/
ICARE Social Media Post January 2021
Sharing Genetic Test Results with Family Members of BRCA, PALB2, CHEK2, and ATM
ICARE Social Media Post January 2021
Sharing Genetic Test Results with Family Members of BRCA, PALB2, CHEK2, and ATM
Check out a new article by the ICARE team, published in Patient Education and Counseling, evaluating the motivators and barriers to sharing personal genetic test results with family members. The article is 𝗳𝗿𝗲𝗲 to access and download 𝘂𝗻𝘁𝗶𝗹 𝗠𝗮𝗿𝗰𝗵 𝟱𝘁𝗵 at: https://www.sciencedirect.com/science/article/pii/S0738399120306832
Permanent link to this article: https://inheritedcancer.net/post11521/
ICARE Publication January 2021
Sharing genetic test results with family members of BRCA, PALB2, CHEK2, and ATM carriers
ICARE Publication January 2021
Sharing genetic test results with family members of BRCA, PALB2, CHEK2, and ATM carriers
Abstract Objective: This study explored motivators and challenges/barriers to sharing personal genetic test results (GTR) with family members (FM). Methods: Semi-structured, in-depth interviews were conducted with 62 women who had a pathogenic or likely pathogenic (P/LP) variant in a BRCA, PALB2, CHEK2, or ATM gene. Selective qualitative data analysis focused on eliciting motivators and challenges/barriers …
Permanent link to this article: https://inheritedcancer.net/pub10521/
ICARE Social Media Post December 2020
Genetic Testing in Women with Breast Cancer
ICARE Social Media Post December 2020
Genetic Testing in Women with Breast Cancer
Approximately 4,000 women with breast cancer were tested for mutations in nine breast cancer genes – 6.2% had mutations in at least one of the nine genes, and 2.7% had mutations in either 𝘽𝙍𝘾𝘼1 or 𝘽𝙍𝘾𝘼2. Comparisons between women who did versus did not meet National Comprehensive Cancer Network (NCCN) guidelines for testing showed that: …
Permanent link to this article: https://inheritedcancer.net/post122920/
Permanent link to this article: https://inheritedcancer.net/post122220/
ICARE Publication December 2020
Contribution of Germline Predisposition Gene Mutations to Breast Cancer Risk in African American Women
ICARE Publication December 2020
Contribution of Germline Predisposition Gene Mutations to Breast Cancer Risk in African American Women
Abstract Background: The risks of breast cancer in African American (AA) women associated with inherited mutations in breast cancer predisposition genes are not well defined. Thus, whether multigene germline hereditary cancer testing panels are applicable to this population is unknown. We assessed associations between mutations in panel-based genes and breast cancer risk in 5054 AA women …
Permanent link to this article: https://inheritedcancer.net/pub121420/
ICARE Social Media Post December 2020
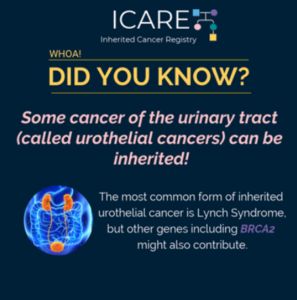
Broader Germline Testing for Urothelial Cancer
ICARE Social Media Post December 2020
Broader Germline Testing for Urothelial Cancer
The most common inherited form of urothelial cancers is Lynch Syndrome. However, a study showed that of 586 individuals with urothelial cancer, 80 had a mutation in an inherited cancer gene (14%). Mutations in several genes were observed; however, 𝙈𝙎𝙃2 and 𝘽𝙍𝘾𝘼2 were both significantly associated with urothelial cancer (odds ratio of 3.7). Confirmatory …
Permanent link to this article: https://inheritedcancer.net/post120420/
Permanent link to this article: https://inheritedcancer.net/post112720/
Permanent link to this article: https://inheritedcancer.net/post112420/
ICARE Social Media Post November 2020
ASCO Guideline Updates: Breast Cancer
ICARE Social Media Post November 2020
ASCO Guideline Updates: Breast Cancer
The American Society of Clinical Oncology (ASCO) published updated guidelines for the management of hereditary breast cancer for the following gene carriers: 𝘽𝙍𝘾𝘼1/2 • Consider breast-conserving therapy • Consider nipple-sparing mastectomy, if medically appropriate • Advanced breast cancer: ⫸ PARP inhibitors (olaparib, talazoparib) preferred over non-platinum single agent chemotherapy ⫸ Platinum agents are recommended …
Permanent link to this article: https://inheritedcancer.net/post112020/
ICARE Social Media Post November 2020
B-GREAT 2020 Newsletter
ICARE Social Media Post November 2020
B-GREAT 2020 Newsletter
The B-GREAT 2020 Newsletter is now available! Check out this latest edition for research updates and information about racial inequalities in healthcare. You can read the newsletter by visiting: https://bgreatinitiative.inheritedcancer.net/wp-content/uploads/BGREAT-Newsletter-2020.pdf. Please feel free to share with family members, friends, and/or your healthcare providers. We will be publishing these newsletters twice a year starting in 2021. …
Permanent link to this article: https://inheritedcancer.net/post111020/
ICARE Publication November 2020
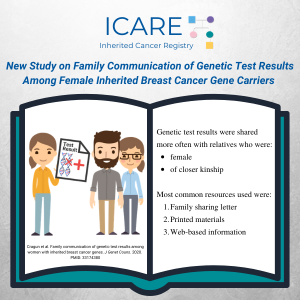
Family communication of genetic test results among women with inherited breast cancer genes
ICARE Publication November 2020
Family communication of genetic test results among women with inherited breast cancer genes
Abstract Identification of inherited breast cancer may guide care. These benefits can be amplified through communication of genetic test results with at-risk family members and subsequent family testing (FT). Females with a pathogenic/likely pathogenic (P/LP) variant in BRCA1/2, PALB2, CHEK2, and/or ATM were surveyed about family communication (FC) of genetic test results and FT. Comparisons …
Permanent link to this article: https://inheritedcancer.net/pub111020/
ICARE Social Media Post November 2020
Clinical Trial Participation Powers Patient’s Positive Attitude
ICARE Social Media Post November 2020
Clinical Trial Participation Powers Patient’s Positive Attitude
Brooke Thomas has leaned on 12 years of experience as a medical social worker and found ways to stay positive and upbeat through it all – and she has a lot to be positive about these days, thanks to an amazing response to her treatment as part of a clinical trial at Vanderbilt-Ingram Cancer Center. …
Permanent link to this article: https://inheritedcancer.net/post110620/
Permanent link to this article: https://inheritedcancer.net/post102720/
ICARE Social Media Post October 2020
New ASCO Guidelines On Use Of PARP Inhibitors To Manage Ovarian Cancer
ICARE Social Media Post October 2020
New ASCO Guidelines On Use Of PARP Inhibitors To Manage Ovarian Cancer
New guidelines for the use of PARP inhibitors to treat ovarian cancer among those with BRCA1 or BRCA2 mutations were published through the American Society of Clinical Oncology (ASCO) to guide providers about the role of this class of drugs in the management of this type of cancer. Link to the guidelines are available at: …
Permanent link to this article: https://inheritedcancer.net/post101320/
Permanent link to this article: https://inheritedcancer.net/video100820/
ICARE Social Media Post October 2020
Addition of Veliparib to Carboplatin/Paclitaxel in Previously Treated Patients With BRCA-Mutated Advanced Breast Cancer
ICARE Social Media Post October 2020
Addition of Veliparib to Carboplatin/Paclitaxel in Previously Treated Patients With BRCA-Mutated Advanced Breast Cancer
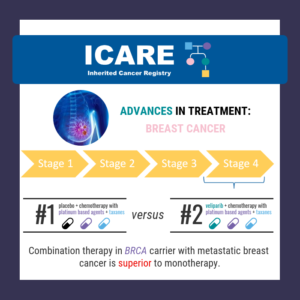
Among BRCA carriers with metastatic breast cancer, the combination of veliparib AND chemotherapy with platinum-based agents (carboplatin) and taxanes (paclitaxel) led to a longer duration of progression free survival (disease that did not progress), compared to those treated with ONLY chemotherapy. To read the full article visit: https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(20)30447-2/fulltext [This finding was previously outlined last year …
Permanent link to this article: https://inheritedcancer.net/post100620/
ICARE Publication September 2020
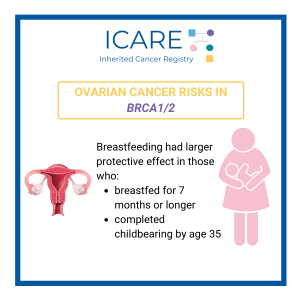
Breastfeeding and the risk of epithelial ovarian cancer among women with a BRCA1 or BRCA2 mutation
ICARE Publication September 2020
Breastfeeding and the risk of epithelial ovarian cancer among women with a BRCA1 or BRCA2 mutation
Abstract Objective: BRCA mutation carriers face a high lifetime risk of developing ovarian cancer. The strong inverse association between breastfeeding and the risk of ovarian cancer is established in the general population but is less well studied among women with a germline BRCA1 or BRCA2 mutation. Method: Thus, we conducted a matched case-control analysis to …
Permanent link to this article: https://inheritedcancer.net/pub93020/
Permanent link to this article: https://inheritedcancer.net/post91820/
ICARE Publication September 2020
Strategies to enhance identification of hereditary breast cancer gene carriers
ICARE Publication September 2020
Strategies to enhance identification of hereditary breast cancer gene carriers
No abstract available Reid S, et al. Strategies to enhance identification of hereditary breast cancer gene carriers. Expert Rev Mol Diagn. 2020 Sep; 20(9):861-865. Epub 2020 Sep 11. PMID: 32856489.
Permanent link to this article: https://inheritedcancer.net/pub91120/
Permanent link to this article: https://inheritedcancer.net/post91020/
ICARE Social Media Post August 2020
Older Women with Breast Cancer Have Higher Risks of Ovarian and Other Cancers
ICARE Social Media Post August 2020
Older Women with Breast Cancer Have Higher Risks of Ovarian and Other Cancers
Among 4,500 post-menopausal women with breast cancer, 3.55% had a mutation in a gene associated with inherited breast cancer (3-fold higher than what was seen among women who were cancer-free). BRCA1/2 mutations were seen more frequently in women diagnosed with breast cancer at or below age 65 (2.21%) compared to those diagnosed after age 65 …
Permanent link to this article: https://inheritedcancer.net/post82820/
ICARE Social Media Post August 2020
MRI Surveillance Has Very Low Yield After Bilateral Mastectomy and Reconstruction
ICARE Social Media Post August 2020
MRI Surveillance Has Very Low Yield After Bilateral Mastectomy and Reconstruction
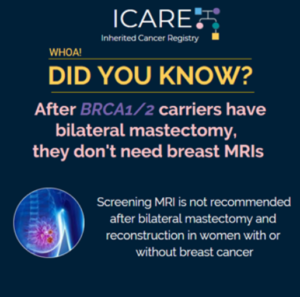
A study of 159 women, including BRCA carriers, who had bilateral mastectomy with reconstruction, underwent breast MRI screening. The results showed few women had detection of breast cancer through MRI, after their bilateral mastectomy. These results support the recommendation against screening MRI in women who have had bilateral mastectomy with reconstruction due to a diagnosis …
Permanent link to this article: https://inheritedcancer.net/post81820/
ICARE Newsletter Summer 2020
Identifying Individuals At-Risk for Inherited Cancer
ICARE Newsletter Summer 2020
Identifying Individuals At-Risk for Inherited Cancer
We have known for a while that many people who have mutations in BRCA1/2 and other inherited cancer risk genes are unaware of their mutation as they have not yet had genetic testing. A recent study among women aged 20 or older living in California and Georgia, which included almost 80,000 breast cancer patients and …
Permanent link to this article: https://inheritedcancer.net/4nls2020/
ICARE Newsletter Summer 2020
Treatment Advances Among BRCA1/2 Carriers
ICARE Newsletter Summer 2020
Treatment Advances Among BRCA1/2 Carriers
There continue to be ongoing advances in treatment studies among those with inherited cancer gene mutations, which are rapidly being followed by FDA approval for specific cancer treatments. Select studies and advances are summarized below: BRCA1/2 Carriers: Breast Cancer: For those with later stage or metastatic breast cancer, the FDA currently has approvals for the use …
Permanent link to this article: https://inheritedcancer.net/2nls2020/
ICARE Newsletter Summer 2020
Guideline-Concordant Care Among Women with Inherited Cancer Gene Mutations
ICARE Newsletter Summer 2020
Guideline-Concordant Care Among Women with Inherited Cancer Gene Mutations
Testing for inherited cancer among breast cancer patients has tremendous potential to guide appropriate care following testing. Yet, a number of efforts suggest that women are not consistently receiving care according to current national guidelines based on their genetic test result. In fact, results from studies suggest many women for whom risk-reducing mastectomy would not …
Permanent link to this article: https://inheritedcancer.net/3nls2020/
Permanent link to this article: https://inheritedcancer.net/post80720/
Permanent link to this article: https://inheritedcancer.net/post73120/
ICARE Social Media Post July 2020
BRCA1/2 and Other Gene Carriers with Breast Cancer Don’t Always Receive Recommended Treatment
ICARE Social Media Post July 2020
BRCA1/2 and Other Gene Carriers with Breast Cancer Don’t Always Receive Recommended Treatment
BRCA1/2 and other gene mutation carriers with early stage breast cancer are not always receiving cancer treatment as recommended by national guidelines. Even though more and more people have been tested for hereditary cancer over the years, using this information accurately to guide treatment has not been as successful. These findings highlight the need for …
Permanent link to this article: https://inheritedcancer.net/post71020/
ICARE Social Media Post June 2020
Advances in Treatment for BRCA-Mutated Triple Negative Breast Cancer
ICARE Social Media Post June 2020
Advances in Treatment for BRCA-Mutated Triple Negative Breast Cancer
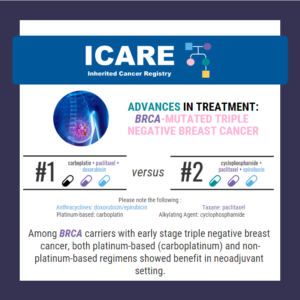
In a study of 914 women with different breast cancer subtypes, overall pathologic complete response rates were: Higher in those with BRCA1/2 mutations (60.4% versus 46.7%) No differences were seen in those with mutations in other inherited cancer genes Among patients with triple-negative breast cancer, BRCA1/2 mutations had highest response rates to treatment in both …
Permanent link to this article: https://inheritedcancer.net/post61920/
Permanent link to this article: https://inheritedcancer.net/post61620/
ICARE Social Media Post June 2020
Advances in BRCA1/2 Breast Cancer Treatment
ICARE Social Media Post June 2020
Advances in BRCA1/2 Breast Cancer Treatment
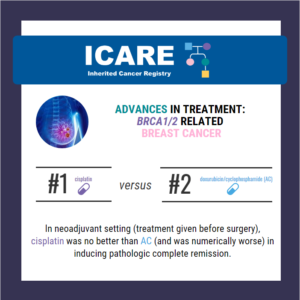
Through a randomized phase 2 study (called the INFORM trial) among BRCA1/2 carriers with breast cancer, cisplatin was no better in inducing pathologic complete remission compared to AC. The pathologic complete remission rate was 18% for cisplatin and 26% for AC. Cisplatin is not better than other chemotherapy for induction therapy for breast cancers in …
Permanent link to this article: https://inheritedcancer.net/post61220/
ICARE Social Media Post June 2020
Advances in Treatment for Pancreatic Cancer: Cisplatin + Gemcitabine
ICARE Social Media Post June 2020
Advances in Treatment for Pancreatic Cancer: Cisplatin + Gemcitabine
In BRCA1/2 or PALB2 carriers with stage 3 or 4 pancreatic cancer, the combination of cisplatin + gemtricitabine with veliparib (a PARP inhibitor), did NOT seem to provide additional benefit over cisplatin + gemtricitabine alone. Through this phase 2 randomized control trial, response rates in both treatment arms were high with similar overall survival rates. …
Permanent link to this article: https://inheritedcancer.net/post60520/
Permanent link to this article: https://inheritedcancer.net/post52920/
ICARE Social Media Post May 2020
Cancer Risk Management Among Female BRCA1/2, PALB2, CHEK2, and ATM Carriers in ICARE
ICARE Social Media Post May 2020
Cancer Risk Management Among Female BRCA1/2, PALB2, CHEK2, and ATM Carriers in ICARE
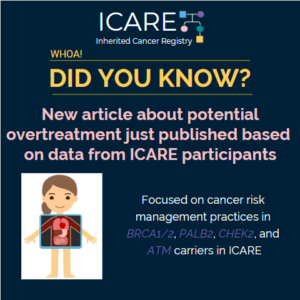
A new article was recently published based on data from BRCA1/2, PALB2, CHEK2, and ATM carriers in ICARE. Findings suggest potential overtreatment through risk-reducing surgery among women with pathogenic/likely pathogenic variants in breast cancer genes. This highlights the importance of promoting guideline-adherent, risk-appropriate care. Check out the full article at https://rdcu.be/b4mbg
Permanent link to this article: https://inheritedcancer.net/post52620/
ICARE Social Media Post May 2020
Advances in Treatment for Metastatic Prostate Cancer: Olaparib
ICARE Social Media Post May 2020
Advances in Treatment for Metastatic Prostate Cancer: Olaparib
On May 19, 2020 the FDA approved the use of olaparib (Lynparza) as treatment in BRCA and other gene carriers (homologous recombination repair genes) with metastatic castration-resistant prostate cancer who have been treated with enzalutamide or abiraterone. Link to full article: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-olaparib-hrr-gene-mutated-metastatic-castration-resistant-prostate-cancer
Permanent link to this article: https://inheritedcancer.net/post52220/
ICARE Publication May 2020
Cancer risk management among female BRCA1/2, PALB2, CHEK2, and ATM carriers
ICARE Publication May 2020
Cancer risk management among female BRCA1/2, PALB2, CHEK2, and ATM carriers
Abstract Purpose: Identification of inherited breast cancer may guide cancer risk management. We sought to compare risk management practices across women with inherited breast cancer genes. Methods: Females with a pathogenic/likely pathogenic (P/LP) variant in BRCA1/2, PALB2, CHEK2, and/or ATM were surveyed about cancer risk management. Comparisons were made across genes. Results: The 235 participants with P/LP variants …
Permanent link to this article: https://inheritedcancer.net/pub52220/
ICARE Social Media Post May 2020
Advances in Treatment for Metastatic Prostate Cancer: Rucaparib
ICARE Social Media Post May 2020
Advances in Treatment for Metastatic Prostate Cancer: Rucaparib
On May 15, 2020 the FDA approved the use of rucaparib (Rubraca) as treatment in BRCA carriers with metastatic castration-resistant prostate cancer who have been treated with androgen receptor-directed therapy and a taxane-based chemotherapy. Link to full article: https://www.fda.gov/drugs/fda-grants-accelerated-approval-rucaparib-brca-mutated-metastatic-castration-resistant-prostate
Permanent link to this article: https://inheritedcancer.net/post51920/
ICARE Social Media Post May 2020
Advances in Ovarian Cancer Treatment for BRCA1/2 Carriers: Olaparib & bevacizumab
ICARE Social Media Post May 2020
Advances in Ovarian Cancer Treatment for BRCA1/2 Carriers: Olaparib & bevacizumab
On May 8, 2020 the FDA approved the use of olaparib (Lynparza) as first-line maintenance treatment in BRCA1/2 carriers (deleterious or suspected deleterious mutations) and/or a genomic instability, with advanced epithelial ovarian, fallopian tube or primary peritoneal cancer who are in complete or partial response to first-line platinum-based chemotherapy. Link to full article: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-olaparib-plus-bevacizumab-maintenance-treatment-ovarian-fallopian-tube-or-primary
Permanent link to this article: https://inheritedcancer.net/post51220/
ICARE Social Media Post May 2020
Advances in Ovarian Cancer Treatment for BRCA1/2 Carriers: Olaparib
ICARE Social Media Post May 2020
Advances in Ovarian Cancer Treatment for BRCA1/2 Carriers: Olaparib
In recognition of World Ovarian Cancer Day, we’d like to share some exciting results from a study of women with ovarian cancer and a BRCA mutation: In a recent phase III trial, olaparib (PARP inhibitor) showed improved response and progression-free survival compared with chemotherapy (without platinum) in BRCA carriers with platinum-sensitive relapsed ovarian cancer who …
Permanent link to this article: https://inheritedcancer.net/post50820/
ICARE Social Media Post May 2020
Advances in Treatment for Metastatic Prostate Cancer: Olaparib
ICARE Social Media Post May 2020
Advances in Treatment for Metastatic Prostate Cancer: Olaparib
Findings from a recent study showed that olaparib (PARP inhibitor) significantly improved progression-free survival in patients with BRCA1, BRCA2, or ATM genetic alterations. Benefits were also more broadly seen among patients with homologous recombination repair gene defects. Link to full article: https://www.nejm.org/doi/full/10.1056/NEJMoa1911440 Check out the ASCO post article at: https://www.ascopost.com/news/may-2020/olaparib-for-patients-with-mcrpc-and-homologous-recombination-repair-gene-alterations/
Permanent link to this article: https://inheritedcancer.net/post50620/
ICARE Social Media Post April 2020
Advances in Treatment for Ovarian Cancer in BRCA1/2 Carriers: Niraparib
ICARE Social Media Post April 2020
Advances in Treatment for Ovarian Cancer in BRCA1/2 Carriers: Niraparib
On April 29, 2020 the FDA approved the use of niraparib (Zeluja) as first-line maintenance treatment in BRCA1/2 carriers with advanced ovarian cancer! More details available at: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-niraparib-first-line-maintenance-advanced-ovarian-cancer
Permanent link to this article: https://inheritedcancer.net/post42920/
ICARE Social Media Post April 2020
BRCA2: Cancer Risks and Risk Management
ICARE Social Media Post April 2020
BRCA2: Cancer Risks and Risk Management
Gene: BRCA2 Cancer Risks and Management (per NCCN version 3.2019): Women: Breast cancer risk: Elevated at 60%-70% – Recommend clinical breast exam every 6-12 months starting at age 25, annual breast MRI with contrast starting at age 25, and annual mammogram with consideration of tomosynthesis starting at age 30; consider risk-reducing mastectomy. Ovarian cancer risk: …
Permanent link to this article: https://inheritedcancer.net/post42120/
ICARE Social Media Post April 2020
ASCO Guideline: Genetic Testing for Ovarian Cancer
ICARE Social Media Post April 2020
ASCO Guideline: Genetic Testing for Ovarian Cancer
The American Society of Clinical Oncology (ASCO) recently published a guideline reinforcing the longstanding recommendation that all women diagnosed with epithelial ovarian cancer (EOC) be offered genetic testing for hereditary ovarian cancer genes. Many of these women (>15%) have an inherited mutation, most commonly BRCA1 or BRCA2. Identifying BRCA1/2 mutations may help guide cancer treatment. …
Permanent link to this article: https://inheritedcancer.net/post41720/
ICARE Newsletter Winter 2020
Treatment Advances Among Those with Inherited Prostate Cancer Predisposition
ICARE Newsletter Winter 2020
Treatment Advances Among Those with Inherited Prostate Cancer Predisposition
A recent study reported a high complete response rate among men with a BRCA1/2 mutation with metastatic, castration-resistant prostate cancer who were treated with niraparib (a PARP inhibitor) of 63% compared to 17% in the non-BRCA1/2 group.1 Based on this data, the Federal Drug Administration (FDA) granted breakthrough therapy designation to niraparib on October 3, …
Permanent link to this article: https://inheritedcancer.net/3nlw2020/
ICARE Newsletter Winter 2020
Treatment Advances Among Those with Inherited Pancreatic Cancer Predisposition
ICARE Newsletter Winter 2020
Treatment Advances Among Those with Inherited Pancreatic Cancer Predisposition
Results from a recent study showed olaparib (a PARP inhibitor) nearly doubled the progression-free survival in BRCA1/2 carriers with metastatic pancreatic cancer.1 Based on this data, the FDA approved the use of olaparib as a first-line maintenance treatment in BRCA1/2 carriers with metastatic, platinum-sensitive pancreatic cancer. This represents another treatment advance in pancreatic cancer and …
Permanent link to this article: https://inheritedcancer.net/2nlw2020/
ICARE Newsletter Winter 2020
Updates to National Comprehensive Cancer Network (NCCN) Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic
ICARE Newsletter Winter 2020
Updates to National Comprehensive Cancer Network (NCCN) Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic
There were significant updates and restructuring of the guidelines, with some highlights included below: Substantial reorganization of the guidelines as follows: Now organized by organ site, rather than primarily by certain high penetrance genes Focused efforts to simplify genetic testing criteria Only one flow diagram included, to outline the ‘genetic testing process’ Following scenarios now …
Permanent link to this article: https://inheritedcancer.net/1nlw2020/
ICARE Newsletter Winter 2020
Updated Pancreatic Cancer Screening Guidelines through CAPS Consortium
ICARE Newsletter Winter 2020
Updated Pancreatic Cancer Screening Guidelines through CAPS Consortium
The International Cancer of the Pancreas Screening (CAPS) Consortium recently published updated recommendations about pancreatic cancer screening through MRI/magnetic retrograde cholangiopancreatography (MRCP) and/or an endoscopic ultrasound (EUS).1 Specifically, these guidelines now recommend that individuals with a CDKN2A or STK11 mutation begin screening at age 40. Screening for individuals with a BRCA1/2, ATM, PALB2, MLH1, or …
Permanent link to this article: https://inheritedcancer.net/4nlw2020/
ICARE Newsletter Winter 2020
Ask the Expert
ICARE Newsletter Winter 2020
Ask the Expert
Through each newsletter, we give our participants an opportunity to have their questions answered by experts. If you have a question you would like addressed, please email the study team at ICARE@InheritedCancer.net for consideration in future newsletters. The following question was addressed by Ben Ho Park, MD, PhD, who is the Donna S. Hall Chair …
Permanent link to this article: https://inheritedcancer.net/8nlw2020/
ICARE Social Media Post February 2020
Ovarian Cancer Risks in BRCA1/2
ICARE Social Media Post February 2020
Ovarian Cancer Risks in BRCA1/2
The risk of ovarian cancer is raised in women with BRCA1/2 mutations. Recent findings suggest that higher body mass index (BMI) may further raise the risk of ovarian cancer in premenopausal BRCA1/2 carriers. Note that all women with BRCA1/2 mutations are at high risk for ovarian cancer, and should follow current National Comprehensive Cancer Network …
Permanent link to this article: https://inheritedcancer.net/post21820/
ICARE Social Media Post February 2020
Characteristics and Patterns of Spread for BRCA-associated Breast Cancers
ICARE Social Media Post February 2020
Characteristics and Patterns of Spread for BRCA-associated Breast Cancers
BRCA1/2 have distinct characteristics and patterns of spread. A recent study evaluated the characteristics of BRCA1/2 carriers and found that 73% ofBRCA1-associated breast cancers were triple negative; and 72% of BRCA2-associated breast cancers were hormone receptor positive. There were also distinct pattern of spread of breast cancer, with BRCA1 carriers more likely to experience lung …
Permanent link to this article: https://inheritedcancer.net/post21320/
ICARE Social Media Post February 2020
Differences in Pancreatic Cancer Screening Recommendations from the National Comprehensive Cancer Network (NCCN) and the International Cancer of the Pancreas Screening (CAPS) Consortium
ICARE Social Media Post February 2020
Differences in Pancreatic Cancer Screening Recommendations from the National Comprehensive Cancer Network (NCCN) and the International Cancer of the Pancreas Screening (CAPS) Consortium
The National Comprehensive Cancer Network (NCCN) and the International Cancer of the Pancreas Screening (CAPS) Consortium recently updated pancreatic cancer screening recommendations. However, there are some differences between these recommendations. Specifically, screening with annual MRI/magnetic retrograde cholangiopancreatography (MRCP) and/or endoscopic ultrasound (EUS) is recommended as follows for NCCN versus CAPS: STK11 regardless of family history: …
Permanent link to this article: https://inheritedcancer.net/post2620/
ICARE Social Media Post February 2020
Updated Pancreatic Cancer Screening Guidelines through the International Cancer of the Pancreas Screening (CAPS) Consortium
ICARE Social Media Post February 2020
Updated Pancreatic Cancer Screening Guidelines through the International Cancer of the Pancreas Screening (CAPS) Consortium
The International Cancer of the Pancreas Screening (CAPS) Consortium recently published updated pancreatic cancer screening recommendations. The recommendations include: Screening with MRI/magnetic retrograde cholangiopancreaography (MRCP) and/or endoscopic ultrasound (EUS) The screening was recommended for the following individuals: CDKN2A and STK11 mutation carriers starting at age 40 BRCA1/2, ATM, PALB2, MLH1, and MSH2 mutation carriers (if …
Permanent link to this article: https://inheritedcancer.net/post2420/
ICARE Social Media Post January 2020
Racial Disparities in Genetic Testing for Women with Ovarian Cancer
ICARE Social Media Post January 2020
Racial Disparities in Genetic Testing for Women with Ovarian Cancer
Women of non-European ancestry diagnosed with ovarian cancer have lower rates of referral for genetic testing despite current national guidelines stating ALL women with ovarian cancer and/or a close-blood relative with ovarian cancer should be offered genetic counseling and testing. One study reported that only 1/3 of Black, Latina, and Asian women were referred for …
Permanent link to this article: https://inheritedcancer.net/post11620/
ICARE Social Media Post January 2020
Women with BRCA1/2: Risk-reducing Mastectomy versus Screening
ICARE Social Media Post January 2020
Women with BRCA1/2: Risk-reducing Mastectomy versus Screening
In a study of 5000 healthy female BRCA1/2 carriers (without a breast cancer diagnosis): BRCA1 carriers: after an average of over 10 years follow-up, survival was higher in those who had bilateral mastectomy (99.7%) compared to those who had breast screening through mammograms and breast MRIs (93%). BRCA2 carriers: no significant differences were seen between …
Permanent link to this article: https://inheritedcancer.net/post11020/
ICARE Social Media Post January 2020
Celebrating 10 Years of ICARE
ICARE Social Media Post January 2020
Celebrating 10 Years of ICARE
Happy New Year! 2020 represents a decade for ICARE We are celebrating 10 years of research, education, and engagement, through which we have enrolled nearly 3500 participants, including over 2000 gene mutation carriers, disseminated 15 newsletters, led and collaborated on multiple research projects, and impacted individuals affected by inherited cancer predisposition all over the …
Permanent link to this article: https://inheritedcancer.net/post1920/
ICARE Social Media Post January 2020
Advances in Treatment for Pancreatic Cancer in BRCA Carriers
ICARE Social Media Post January 2020
Advances in Treatment for Pancreatic Cancer in BRCA Carriers
The FDA approved the use of olaparib, a PARP inhibitor, as first-line maintenance treatment in BRCA1/2 carriers with metastatic, platinum-sensitive, pancreatic cancer. Platinum-sensitive cancer is a cancer that responds to treatment with drugs that contain the metal platinum, such as carboplatin or cisplatin. Olaparib showed to nearly double the progression-free survival in BRCA1/2 carriers with …
Permanent link to this article: https://inheritedcancer.net/post1320/
ICARE Social Media Post December 2019
Evaluation of PARP Inhibitors in BRCA-Associated Prostate Cancer
ICARE Social Media Post December 2019
Evaluation of PARP Inhibitors in BRCA-Associated Prostate Cancer
The FDA granted breakthrough therapy designation to niraparib (a PARP inhibitor) for the treatment of men with BRCA1/2 positive, metastatic castration-resistant, and heavily pre-treated prostate cancer. Results from a recent study show a 63% complete response rate in men with BRCA1/2 positive, metastatic castration-resistant prostate cancer treated with niraparib compared to 17% in the non- …
Permanent link to this article: https://inheritedcancer.net/post122019/
ICARE Publication December 2019
Points to consider: is there evidence to support BRCA1/2 and other inherited breast cancer genetic testing for all breast cancer patients? A statement of the American College of Medical Genetics and Genomics (ACMG)
ICARE Publication December 2019
Points to consider: is there evidence to support BRCA1/2 and other inherited breast cancer genetic testing for all breast cancer patients? A statement of the American College of Medical Genetics and Genomics (ACMG)
No abstract available Pal T, et al. Points to consider: is there evidence to support BRCA1/2 and other inherited breast cancer genetic testing for all breast cancer patients? A statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2020 Apr;22(4):681-685. Epub 2019 Dec 13. PMID: 33455826.
Permanent link to this article: https://inheritedcancer.net/pub121319/
ICARE Social Media Post December 2019
Patient Reported Outcomes In A Study of PARP Inhibitors in BRCA Carriers with Metastatic Breast Cancer
ICARE Social Media Post December 2019
Patient Reported Outcomes In A Study of PARP Inhibitors in BRCA Carriers with Metastatic Breast Cancer
Did you know? PROs are impacting treatment advances in metastatic breast cancer. Olaparib increased progression-free survival among BRCA carriers with metastatic HER2- breast cancer. Thanks to patient reported outcomes, a new study now suggests it also improved patients’ quality of life! Check it out the new article published in October 2019 directly at https://www.ncbi.nlm.nih.gov/pubmed/31446213!
Permanent link to this article: https://inheritedcancer.net/post12819/
ICARE Social Media Post December 2019
Updates to National Comprehensive Cancer Network (NCCN) Genetic/Familial Breast, Ovarian, and Pancreatic Guidelines (V1.2020)
ICARE Social Media Post December 2019
Updates to National Comprehensive Cancer Network (NCCN) Genetic/Familial Breast, Ovarian, and Pancreatic Guidelines (V1.2020)
We are excited to share the latest version of the NCCN Genetic/Familial Breast, Ovarian and Pancreatic Guidelines (V1.2020), which were just updated. Some of the changes made include: PALB2 was added as a high penetrance gene (similar to BRCA1, BRCA2, CDH1, PTEN and TP53) It is appropriate to consider risk reducing mastectomy for cancer risk management …
Permanent link to this article: https://inheritedcancer.net/post12419/
ICARE Social Media Post November 2019
High Frequency of BRCA in Unselected Women with Metastatic Breast Cancer
ICARE Social Media Post November 2019
High Frequency of BRCA in Unselected Women with Metastatic Breast Cancer
Did you know? National practice guidelines currently recommend ALL women with metastatic (HER2-) breast cancer to get genetic testing for inherited cancer (including BRCA1/2 testing), because it can guide eligibility for treatment with PARP inhibitors. A new study led by our colleague at the Vanderbilt-Ingram Cancer Center, Dr. Ben Park, suggests that more women with …
Permanent link to this article: https://inheritedcancer.net/post110719/
ICARE Publication November 2019
Impact of Genetic Testing on Risk-Management Behavior of Black Breast Cancer Survivors: A Longitudinal, Observational Study
ICARE Publication November 2019
Impact of Genetic Testing on Risk-Management Behavior of Black Breast Cancer Survivors: A Longitudinal, Observational Study
Abstract Background: Black women are overrepresented among premenopausal breast cancer (BC) survivors. These patients warrant genetic testing (GT) followed by risk-reducing behaviors. This study documented patterns and predictors of cancer risk-management behaviors among young black BC survivors after GT. Methods: Black women (n = 143) with a diagnosis of BC at the age of 50 years or …
Permanent link to this article: https://inheritedcancer.net/pub110119/
ICARE Social Media Post October 2019
Lifestyle Factors Associated with Familial and Inherited Breast Cancer Risks
ICARE Social Media Post October 2019
Lifestyle Factors Associated with Familial and Inherited Breast Cancer Risks
Body mass index (BMI) is an indicator of fat content in the body. A lower body mass index may reduce breast cancer risk in women, including those with a family history of breast cancer. However, a new study reported that higher BMI also increases breast cancer risk in the general population and those with a …
Permanent link to this article: https://inheritedcancer.net/post102919/
ICARE Social Media Post October 2019
Physical Activity Associated with Familial and Inherited Breast Cancer Risks
ICARE Social Media Post October 2019
Physical Activity Associated with Familial and Inherited Breast Cancer Risks
BRCA1/2 carriers may benefit from physical activity to reduce breast cancer risks, just like women in the general population! We know that women in the general population benefit from physical activity to reduce their breast cancer risk, but a recent study showed that this benefit also extends to those with a BRCA1/2 mutation and those with …
Permanent link to this article: https://inheritedcancer.net/post102819/
ICARE Social Media Post October 2019
BRCA Testing In Black Women
ICARE Social Media Post October 2019
BRCA Testing In Black Women
Did you know? Although BRCA testing has been around for over TWO decades, not all populations have benefitted equally from testing. In fact, our previous research has shown that black patients are less aware of BUT interested in genetic testing…when they know about it. In addition, healthcare providers are less likely to identify and suggest …
Permanent link to this article: https://inheritedcancer.net/post102419/
ICARE Social Media Post October 2019
Advances in Treatment for Advanced Breast Cancer in BRCA Carriers
ICARE Social Media Post October 2019
Advances in Treatment for Advanced Breast Cancer in BRCA Carriers
Monotherapy with PARP inhibitors is FDA-approved for patients with metastatic breast cancer with BRCA mutations. BUT, does adding additional drugs (called ‘combination therapy’) help? In BRCA carriers with metastatic breast cancer, the combination of veliparib AND chemotherapy with platinum-based agents (carboplatin) and taxanes (paclitaxel) led to a longer duration of progression free survival (disease that …
Permanent link to this article: https://inheritedcancer.net/post101819/
ICARE Social Media Post October 2019
Advances in Early Stage Breast Cancer Treatment for BRCA Carriers
ICARE Social Media Post October 2019
Advances in Early Stage Breast Cancer Treatment for BRCA Carriers
New benefits from a PARP inhibitor, talazoparib, among BRCA carriers with early stage breast cancer. The current FDA approvals for the use of PARP inhibitors is limited to women with metastatic (stage IV) breast cancer. These drugs are being tested in early stage breast cancer to shrink down the tumor (called neoadjuvant treatment) before surgery …
Permanent link to this article: https://inheritedcancer.net/post101519/
ICARE Social Media Post October 2019
Male Breast Cancer Risk
ICARE Social Media Post October 2019
Male Breast Cancer Risk
Did you know? Beyonce’s father, Matthew Knowles, was diagnosed with breast cancer. He states, “we used to think this was only an issue for women, but this is male or female.” According to CBS news, “he is hoping that sharing his story as man with breast cancer will shine a light on the risk men …
Permanent link to this article: https://inheritedcancer.net/post10619/
ICARE Newsletter Summer 2019
Ask the Expert
ICARE Newsletter Summer 2019
Ask the Expert
The following question was addressed by Gillian Hooker, PhD, ScM, LCGC, who is the president-elect for the National Society of Genetic Counselors, Adjunct Associate Professor in the Division of Genetic Medicine at the Vanderbilt University Medical Center, and the Vice President of Clinical Development for Concert Genetics in Nashville, TN. Q. Why was the BRCA1/2 …
Permanent link to this article: https://inheritedcancer.net/10nls2019/
ICARE Newsletter Summer 2019
Ovarian Cancer Treatment Advances for BRCA1/2 Carriers
ICARE Newsletter Summer 2019
Ovarian Cancer Treatment Advances for BRCA1/2 Carriers
A recently reported study of women with ovarian cancer and homologous recombination deficiency (HRD) who received a PARP inhibitor (niraparib) as fourth line or later treatment showed potential clinical benefit. Specifically, median overall survival after treatment was 19 months in the HRD-positive group (including those with BRCA1/2 mutations) compared to 15.5 months in the HRD-negative …
Permanent link to this article: https://inheritedcancer.net/4nls2019/
ICARE Newsletter Summer 2019
New Information About Cancer Risks for Inherited Cancer Genes: BRCA1/2
ICARE Newsletter Summer 2019
New Information About Cancer Risks for Inherited Cancer Genes: BRCA1/2
Looking at pregnancy history and breast cancer risk, a recent study of almost 8,000 women with BRCA1/2 mutations evaluated breast cancer risks related to pregnancy.1 Findings suggested the overall number of pregnancies was not associated with breast cancer risk in BRCA1 carriers; however, BRCA1 carriers with one pregnancy were at higher risk for breast cancer …
Permanent link to this article: https://inheritedcancer.net/6nls2019/
ICARE Newsletter Summer 2019
Pancreatic Cancer Treatment Advances for BRCA1/2 Carriers
ICARE Newsletter Summer 2019
Pancreatic Cancer Treatment Advances for BRCA1/2 Carriers
Results from a clinical trial of individuals with a BRCA1/2 mutation and pancreatic cancer showed that patients who received a PARP inhibitor (olaparib) for maintenance treatment had almost half the risk of their disease progressing when compared to receiving a placebo.1 In fact, after 2 years, 22.1% of patients who received olaparib had no disease …
Permanent link to this article: https://inheritedcancer.net/3nls2019/
ICARE Newsletter Summer 2019
Prostate Cancer Treatment Advances for BRCA1/2 Carriers
ICARE Newsletter Summer 2019
Prostate Cancer Treatment Advances for BRCA1/2 Carriers
There is now information to suggest that identifying inherited mutations in DNA repair genes, such as BRCA1/2 and other genes, in men with metastatic prostate cancer may open doors for other treatment options. Results of a phase 2 clinical trial among men with metastatic and heavily pre-treated prostate cancer were presented at the American Society …
Permanent link to this article: https://inheritedcancer.net/2nls2019/
ICARE Newsletter Summer 2019
Expansion of Criteria for BRCA1/2 Testing through the USPSTF
ICARE Newsletter Summer 2019
Expansion of Criteria for BRCA1/2 Testing through the USPSTF
The U.S. Preventive Services Task Force (USPSTF) came out with new genetic testing guidelines for the BRCA1/2 genes, which has garnered substantial media attention. This task force consists of a team of primary care and preventive medicine healthcare experts to lower the chance of a conflict of interest (which is also the reason that subspecialty …
Permanent link to this article: https://inheritedcancer.net/5nls2019/
ICARE Publication April 2019
International trends in the uptake of cancer risk reduction strategies in women with a BRCA1 or BRCA2 mutation
ICARE Publication April 2019
International trends in the uptake of cancer risk reduction strategies in women with a BRCA1 or BRCA2 mutation
Abstract Background: Women with a BRCA1 or BRCA2 mutation face high risks of breast and ovarian cancer. In the current study, we report on uptake of cancer screening and risk-reduction options in a cohort of BRCA mutation carriers from ten countries over two time periods (1995 to 2008 and 2009 to 2017). Methods: Eligible subjects were identified …
Permanent link to this article: https://inheritedcancer.net/pub41119/
ICARE Publication April 2019
A randomized controlled intervention to promote readiness to genetic counseling for breast cancer survivors
ICARE Publication April 2019
A randomized controlled intervention to promote readiness to genetic counseling for breast cancer survivors
Abstract Objective: Breast cancer (BC) survivors with a genetic mutation are at higher risk for subsequent cancer; knowing genetic risk status could help survivors make decisions about follow-up screening. Uptake of genetic counseling and testing (GC/GT) to determine BRCA status is low among high risk BC survivors. This study assessed feasibility, acceptability, and preliminary efficacy of …
Permanent link to this article: https://inheritedcancer.net/pub41119-2/
ICARE Newsletter Winter 2019
Basal Cell Cancers May Be a Risk Factor to Predict Inherited Cancer Predisposition
ICARE Newsletter Winter 2019
Basal Cell Cancers May Be a Risk Factor to Predict Inherited Cancer Predisposition
An interesting area of progress to identify individuals with inherited risks included a study of over 13,000 individuals with six or more basal cell cancers (BCC) evaluated through a claims database. Results indicated ~20% of these individuals had a germline mutation in a DNA repair gene, including BRCA1/2, PALB2, and the Lynch syndrome genes, among …
Permanent link to this article: https://inheritedcancer.net/10nlw2019/
ICARE Newsletter Winter 2019
New Research and Approvals of PARP Inhibitor Drugs to Treat Prostate Cancer in BRCA Carriers
ICARE Newsletter Winter 2019
New Research and Approvals of PARP Inhibitor Drugs to Treat Prostate Cancer in BRCA Carriers
Treatment among patients with metastatic castration-resistant prostate cancer: A PARP inhibitor (rucaparib) was granted a breakthrough therapy designation in October 2018 for monotherapy (i.e., sole treatment) among men with metastatic castration-resistant prostate cancer (with a BRCA1/2 mutation) who have received at least one prior androgen receptor-directed treatment and taxane-based chemotherapy. This designation was granted based …
Permanent link to this article: https://inheritedcancer.net/3nlw2019/
ICARE Newsletter Winter 2019
Ask the Expert
ICARE Newsletter Winter 2019
Ask the Expert
The following question was addressed by Georgia Wiesner, MD, MS, a nationally renowned clinical cancer geneticist, who is an Ingram Professor of Cancer Research, Professor of Medicine in the Division of Genetic Medicine, and the Director of the Clinical and Translational Hereditary Cancer Program for the Vanderbilt-Ingram Cancer Center in Nashville, Tennessee. Q. What are …
Permanent link to this article: https://inheritedcancer.net/14nlw2019/
ICARE Newsletter Winter 2019
New Research and Approvals of PARP Inhibitor Drugs to Treat Breast Cancer in BRCA Carriers
ICARE Newsletter Winter 2019
New Research and Approvals of PARP Inhibitor Drugs to Treat Breast Cancer in BRCA Carriers
Treatment among patients with advanced or metastatic breast cancer: A PARP inhibitor (talazoparib) was approved by the FDA on October 16, 2018 for BRCA carriers with HER2-negative locally advanced or metastatic breast cancer, based on results of the EMBRACA trial outlined in the last ICARE newsletter. Litton JK, et al. N Engl J Med. 2018 …
Permanent link to this article: https://inheritedcancer.net/1nlw2019/
ICARE Newsletter Winter 2019
Refining Treatment for Aggressive Prostate Cancer in Men with BRCA2
ICARE Newsletter Winter 2019
Refining Treatment for Aggressive Prostate Cancer in Men with BRCA2
A recent study reported that a large proportion of men with aggressive prostate cancer have inherited cancer gene mutations. Specifically, among 400 patients with castration-resistant prostate cancer, 16.2% had a germline mutation in a DNA damage repair gene, including 3% with a BRCA2 mutation. Among BRCA2 carriers, survival was 17.4 months, which was much lower …
Permanent link to this article: https://inheritedcancer.net/5nlw2019/
ICARE Newsletter Winter 2019
New Research and Approvals of PARP Inhibitor Drugs to Treat Ovarian Cancer in BRCA Carriers
ICARE Newsletter Winter 2019
New Research and Approvals of PARP Inhibitor Drugs to Treat Ovarian Cancer in BRCA Carriers
First line maintenance treatment among patients newly diagnosed with advanced ovarian cancer: The results of a trial using a PARP inhibitor (olaparib) as maintenance treatment among ovarian cancer patients with advanced disease, a BRCA mutation, and complete or partial response to platinum-based chemotherapy showed that survival at 3 years was 60% among those who got …
Permanent link to this article: https://inheritedcancer.net/2nlw2019/
ICARE Newsletter Winter 2019
Testing Interpretation and Variant Reclassification
ICARE Newsletter Winter 2019
Testing Interpretation and Variant Reclassification
Results of germline genetic testing generally yield three types of test results: Deleterious (positive), Negative (no mutation detected), and Variant of Uncertain Significance (VUS). As more genes are tested, the chance for a positive result goes up, as does the chance of receiving a VUS result.1 VUS results tell us that it remains uncertain whether …
Permanent link to this article: https://inheritedcancer.net/15nlw2019/
ICARE Newsletter Winter 2019
New Online Risk Calculator to More Accurately Predict Breast Cancer Risk
ICARE Newsletter Winter 2019
New Online Risk Calculator to More Accurately Predict Breast Cancer Risk
Prediction of breast cancer risk is important to identify those at highest and lowest risks, to help guide screening. A previously developed risk algorithm called Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA) was recently extended to include truncating mutations in the BRCA genes, PALB2, CHEK2, and ATM. This online risk …
Permanent link to this article: https://inheritedcancer.net/11nlw2019/
ICARE Newsletter Summer 2018
The Role of Inherited Genes Increasingly Recognized in Pancreatic Cancer
ICARE Newsletter Summer 2018
The Role of Inherited Genes Increasingly Recognized in Pancreatic Cancer
A number of recent studies have suggested that a substantial number of individuals with pancreatic cancer have a mutation in an inherited cancer gene. In a study of over 300 patients with pancreatic cancer (and with one or two family members with pancreatic cancer), 12% were found to have a mutation in 1 of 11 …
Permanent link to this article: https://inheritedcancer.net/4nls2018/
ICARE Newsletter Summer 2018
Community Spotlight
ICARE Newsletter Summer 2018
Community Spotlight
I was aware from a very young age that breast cancer was part of our family. I knew that my great-grandmother (whom I never met) had breast cancer and my grandmother was diagnosed in her 50’s. While I didn’t grow up being afraid of the disease, I was far more aware of it than were …
Permanent link to this article: https://inheritedcancer.net/spotlightnls2018/
ICARE Newsletter Summer 2018
Another PARP-Inhibitor Trial Among BRCA Carriers with Advanced Breast Cancer
ICARE Newsletter Summer 2018
Another PARP-Inhibitor Trial Among BRCA Carriers with Advanced Breast Cancer
In a Phase 3 clinical trial among BRCA carriers with advanced breast cancer, an oral PARP Inhibitor (talazoparib) was compared to standard chemotherapy. Among those who received the PARP inhibitor, risk of disease progression or death was 46% lower, and the response rate was double. Furthermore, the side effect profile, quality-of-life measures, and breast cancer …
Permanent link to this article: https://inheritedcancer.net/8nls2018/
ICARE Newsletter Summer 2018
Ask the Expert
ICARE Newsletter Summer 2018
Ask the Expert
The following question was addressed by Ronald D. Alvarez, MD, MBA who is Professor, Chairman, and Clinical Service Chief of the Department of Obstetrics and Gynecology at Vanderbilt University Medical Center in Nashville, Tennessee. Dr. Alvarez has been the recipient of several National Cancer Institute (NCI) and other industry funded grants in support of his …
Permanent link to this article: https://inheritedcancer.net/10nls2018/
ICARE Newsletter Winter 2018
Community Spotlight
ICARE Newsletter Winter 2018
Community Spotlight
A cancer diagnosis is a life-changing event for every patient and their extended family. However, how we respond to this diagnosis are as individual as our very existence as evidenced by our looks and personalities. Following my diagnosis of stage 4 prostate cancer in 2014 at age 54 which had spread to my bones, I …
Permanent link to this article: https://inheritedcancer.net/spotlightnlw2018/
ICARE Newsletter Winter 2018
Advances in the Understanding of Inherited Prostate Cancer
ICARE Newsletter Winter 2018
Advances in the Understanding of Inherited Prostate Cancer
Findings through a recent study reported that inherited cancer gene mutations were present in 8.2% of those with advanced or metastatic prostate cancer, which provides additional support to include this group of men in broader testing, particularly as targeted treatments based on inherited gene mutations becomes increasingly available.1 Another recent study suggested that those with …
Permanent link to this article: https://inheritedcancer.net/10nlw2018/
ICARE Newsletter Winter 2018
Updates to NCCN Genetic/Familial High-Risk Assessment: Breast and Ovarian Guidelines
ICARE Newsletter Winter 2018
Updates to NCCN Genetic/Familial High-Risk Assessment: Breast and Ovarian Guidelines
(Version 1.2018, posted Oct. 3, 2017) Metastatic prostate cancer was added as an indication for evaluation and testing for the BRCA1 and BRCA2 genes Among BRCA1, BRCA2, TP53 and PTEN carriers, women between ages 25-29 may consider having an annual mammogram with consideration of tomosynthesis if a breast MRI is not available. Among female BRCA2 …
Permanent link to this article: https://inheritedcancer.net/1nlw2018/
ICARE Newsletter Winter 2018
Refining Risks and Outcomes of Breast Cancer in BRCA Carriers
ICARE Newsletter Winter 2018
Refining Risks and Outcomes of Breast Cancer in BRCA Carriers
In an effort to further study breast cancer risks among BRCA carriers, a recently published study compared breast cancer risks among those with and without a close family member (first-degree relative) with breast cancer.1 Findings showed that risk for breast cancer by age 80 was 60.8% in BRCA1 carriers and 63.1% among BRCA2 carriers, with …
Permanent link to this article: https://inheritedcancer.net/4nlw2018/
ICARE Newsletter Winter 2018
FDA Approval of PARP Inhibitor (Lynparza) for Treatment of Advanced Breast Cancer
ICARE Newsletter Winter 2018
FDA Approval of PARP Inhibitor (Lynparza) for Treatment of Advanced Breast Cancer
On January 12, 2018, the FDA approved the first PARP Inhibitor (Lynparza) for treatment in patients with advanced breast cancer due to inherited BRCA mutations.1 This drug is already approved for certain BRCA carriers for advanced ovarian cancer. PARP inhibitors were originally developed to target the specific pathway through which cancer develops among those with …
Permanent link to this article: https://inheritedcancer.net/6nlw2018/
ICARE Newsletter Winter 2018
Ask the Expert
ICARE Newsletter Winter 2018
Ask the Expert
The following question was addressed by Dr. Ingrid Meszoely is a breast surgeon and Clinical Director of the Vanderbilt Breast Center at One Hundred Oaks. She leads a high-risk clinic, through which she and her team of nurse practitioners manage patients with inherited breast cancer predisposition. Her research interests include both clinical and translational breast …
Permanent link to this article: https://inheritedcancer.net/8nlw2018/
ICARE Newsletter Winter 2018
Study Suggests Inherited Cancer Genes Are Important in Pancreatic Cancer
ICARE Newsletter Winter 2018
Study Suggests Inherited Cancer Genes Are Important in Pancreatic Cancer
In a recent study which included over 800 patients with pancreatic ductal cancer, inherited cancer gene mutations were found in a much higher proportion than expected. Almost 5% of these patients had mutations identified in inherited cancer genes, the majority of which were in genes thought to be associated with pancreatic cancer (including BRCA2, ATM, …
Permanent link to this article: https://inheritedcancer.net/9nlw2018/
ICARE Newsletter Summer 2017
Ask the Expert
ICARE Newsletter Summer 2017
Ask the Expert
The following question was addressed by Dr. Steven Narod who is a Tier I Canada Research Chair in Breast Cancer and a senior scientist at the Women’s College Research Institute in Toronto, Canada. Dr. Narod is a world-leader in the field of breast and ovarian cancer genetics. Q. In women with a BRCA mutation and …
Permanent link to this article: https://inheritedcancer.net/6nls2017/
ICARE Newsletter Summer 2017
Breast and Ovarian Cancer Risks Among BRCA Carriers Followed Over Time
ICARE Newsletter Summer 2017
Breast and Ovarian Cancer Risks Among BRCA Carriers Followed Over Time
Findings from an international study of over 6000 women with a BRCA1 mutation and almost 4000 women with a BRCA2 mutation followed for an average of 5 years were recently published.1 Results showed the risk of breast cancer by age 80 was ~70% for both BRCA1 and BRCA2 carriers. Rates of breast cancer increased until …
Permanent link to this article: https://inheritedcancer.net/1nls2017/
ICARE Newsletter Summer 2017
Community Spotlight
ICARE Newsletter Summer 2017
Community Spotlight
After a long rainy summer filled with doctor visits, I was finally diagnosed with triple-negative inflammatory breast cancer (TN IBC) at the age of 49. I completed treatment in June 2008 and was grateful to have a new phase to my vocabulary – NED, ‘no evidence of disease’. Since there was no cancer history in my …
Permanent link to this article: https://inheritedcancer.net/spotlightnls2017/
ICARE Newsletter Summer 2017
New Results of a PARP Inhibitor Study Among BRCA Carriers with Metastatic Breast Cancer
ICARE Newsletter Summer 2017
New Results of a PARP Inhibitor Study Among BRCA Carriers with Metastatic Breast Cancer
Over the last decade, a new class of drugs called “PARP Inhibitors” has been evaluated as a form of targeted treatment among BRCA carriers. Results were recently reported from a Phase 3 clinical trial among BRCA carriers with HER2-negative metastatic breast cancer who received two or less prior chemotherapy regimens for their metastatic disease. Study …
Permanent link to this article: https://inheritedcancer.net/4nls2017/
ICARE Newsletter Summer 2017
What Are the Benefits of Adding a Mammogram to MRI for Breast Cancer Screening Among Women with BRCA Mutations?
ICARE Newsletter Summer 2017
What Are the Benefits of Adding a Mammogram to MRI for Breast Cancer Screening Among Women with BRCA Mutations?
Recently, researchers evaluated the benefit of adding a mammogram to MRI for breast cancer screening among ~2000 women with a BRCA1 or BRCA2 mutation. Results indicated that the addition of mammography to MRI did not substantially raise the chance of detecting breast cancer in the overall group. However, one-third of breast cancer cases diagnosed among …
Permanent link to this article: https://inheritedcancer.net/7nls2017/
Permanent link to this article: https://inheritedcancer.net/2nls2017/
ICARE Newsletter Winter 2017
Newly Approved PARP-Inhibitor (Rucaparib) to Treat BRCA Carriers with Ovarian Cancer
ICARE Newsletter Winter 2017
Newly Approved PARP-Inhibitor (Rucaparib) to Treat BRCA Carriers with Ovarian Cancer
The FDA just approved another PARP inhibitor, rucaparib, for BRCA carriers with ovarian cancer who have already been treated with two or more chemotherapies. Among those with BRCA-mutant ovarian cancers, 54% had a partial or complete response to the drug with a median duration response of 9.2 months. The agency also approved a companion diagnostic …
Permanent link to this article: https://inheritedcancer.net/6nlw2017/
ICARE Newsletter Winter 2017
New Study Suggesting BRCA1/2 and ATM Are Associated with Aggressive Prostate Cancer
ICARE Newsletter Winter 2017
New Study Suggesting BRCA1/2 and ATM Are Associated with Aggressive Prostate Cancer
Among 799 patients with prostate cancer, the rate of BRCA1/2 mutations was much higher among those who passed away of prostate cancer (6.07%) compared to those with low risk disease (1.44%).1 Among the group that died of prostate cancer, those with BRCA1/2 or ATM mutations passed away at an earlier age and had a shorter …
Permanent link to this article: https://inheritedcancer.net/3nlw2017/
ICARE Newsletter Winter 2017
Ask the Expert
ICARE Newsletter Winter 2017
Ask the Expert
The following question was addressed by Dr. Steven Narod who is a Tier I Canada Research Chair in Breast Cancer and a senior scientist at the Women’s College Research Institute in Toronto, Canada. Dr. Narod is a world-leader in the field of breast and ovarian cancer genetics. Q. Does salpingo-oophorectomy reduce the risk of breast …
Permanent link to this article: https://inheritedcancer.net/7nlw2017/
ICARE Newsletter Winter 2017
How Does Having a Mother with Breast Cancer and a BRCA Mutation Affect Adolescent Girls?
ICARE Newsletter Winter 2017
How Does Having a Mother with Breast Cancer and a BRCA Mutation Affect Adolescent Girls?
A recent study compared psychosocial adjustment and risk perception among 11 to 19 year old daughters of women with breast cancer, comparing those with a BRCA mutation versus those without.1 The overall findings from the study were reassuring, suggesting that adolescent girls from BRCA-positive families had higher self-esteem and similar psychosocial adjustment compared to their …
Permanent link to this article: https://inheritedcancer.net/5nlw2017/
ICARE Newsletter Summer 2016
What Are the Endometrial Cancer Risks Among BRCA Carriers?
ICARE Newsletter Summer 2016
What Are the Endometrial Cancer Risks Among BRCA Carriers?
Although BRCA mutations confer increased risk for ovarian, fallopian tube, and primary peritoneal cancer, there have been limited and conflicting risks reported for endometrial cancer. Consequently, current practice guidelines only recommend the removal of the fallopian tubes and ovaries as a risk-reducing option for BRCA carriers.1 Specifically, through a prospective study of 4500 women with …
Permanent link to this article: https://inheritedcancer.net/9nls2016/
ICARE Newsletter Summer 2016
Ask the Expert
ICARE Newsletter Summer 2016
Ask the Expert
The following question was addressed by Dr. Christine Laronga at the Moffitt Cancer Center: Q. How should bone health be monitored in women with a BRCA mutation after removal of the ovaries (i.e., risk-reducing salpingo-oophorectomy (RRSO))? A. Women with a BRCA mutation have a substantially high risk to develop ovarian cancer in their lifetime, yet …
Permanent link to this article: https://inheritedcancer.net/10nls2016/
ICARE Newsletter Summer 2016
Inherited Cancer Genes and Metastatic Prostate Cancer
ICARE Newsletter Summer 2016
Inherited Cancer Genes and Metastatic Prostate Cancer
Several prior studies have suggested that men with a BRCA mutation (primarily BRCA2) tend to develop an aggressive form of prostate cancer that is more likely to metastasize. These findings were recently extended through a new study published in the New England Journal of Medicine.1 In this study, almost 700 men with metastatic prostate cancer, …
Permanent link to this article: https://inheritedcancer.net/5nls2016/
ICARE Newsletter Winter 2016
How Much Does Age at First Breast Cancer Affect the Risk of Contralateral Breast Cancer Risk in BRCA Carriers?
ICARE Newsletter Winter 2016
How Much Does Age at First Breast Cancer Affect the Risk of Contralateral Breast Cancer Risk in BRCA Carriers?
A recently published study of Dutch patients (including 200 BRCA1 carriers, 71 BRCA2 carriers, and 6023 non-carriers) showed that the contralateral breast cancer (breast cancer in the opposite breast) risks at 10 years was 21.1% for BRCA1, 10.8% for BRCA2, and 5.1% for non-carriers. Among BRCA mutation carriers, it was important to take age at …
Permanent link to this article: https://inheritedcancer.net/3nlw2016/
ICARE Newsletter Winter 2016
Ask the Expert
ICARE Newsletter Winter 2016
Ask the Expert
The following question was addressed by Dr. Christine Laronga at the Moffitt Cancer Center: Q. As a BRCA carrier, is it reasonable for me to consider nipple-sparing mastectomy (compared to total mastectomy) to reduce my future risks of breast cancer? A. One strategy to manage the high (60-70%) lifetime breast cancer risk among women with …
Permanent link to this article: https://inheritedcancer.net/6nlw2016/
ICARE Newsletter Winter 2016
Potential Use of PARP-Inhibitors Among Men with Prostate Cancer Who Carry a Mutation in BRCA or Other DNA-Repair Gene
ICARE Newsletter Winter 2016
Potential Use of PARP-Inhibitors Among Men with Prostate Cancer Who Carry a Mutation in BRCA or Other DNA-Repair Gene
A recent study published in the New England Journal of Medicine suggests that PARP-Inhibitors may be of potential use in men who are no longer responding to standard treatments and carry either somatic (i.e., tumor) and/or germline (inherited) mutations in DNA-repair genes (i.e., BRCA1/2, ATM, Fanconi Anemia genes and CHEK2).1 Of 49 men with prostate …
Permanent link to this article: https://inheritedcancer.net/4nlw2016/
ICARE Newsletter Winter 2016
The Importance of Sharing Genetic Test Results with Family Members
ICARE Newsletter Winter 2016
The Importance of Sharing Genetic Test Results with Family Members
Once an individual has had genetic testing for inherited cancer predisposition this information could help their close family members. For example, when a BRCA mutation or a mutation in another inherited cancer gene is found, it is important for close family members (with or without a diagnosis of cancer) to know so they too can …
Permanent link to this article: https://inheritedcancer.net/1nlw2016/
ICARE Newsletter Winter 2016
More Information About the Inherited Component of Pancreatic Cancer
ICARE Newsletter Winter 2016
More Information About the Inherited Component of Pancreatic Cancer
Although pancreatic cancer is one of the cancer types seen among individuals with mutations in inherited cancer genes (including BRCA2 and BRCA1), the proportion of individuals with pancreatic cancer who have an inherited cause has remained uncertain. To further clarify the role of BRCA1 and BRCA2 (BRCA), over 300 patients with pancreatic cancer were tested …
Permanent link to this article: https://inheritedcancer.net/7nlw2016/
ICARE Newsletter Summer 2015
Location and Type of BRCA1/2 Mutation May Impact Breast and Ovarian Cancer Risks
ICARE Newsletter Summer 2015
Location and Type of BRCA1/2 Mutation May Impact Breast and Ovarian Cancer Risks
A study of almost 20,000 BRCA1 carriers and 12,000 BRCA2 carriers demonstrated differences in breast and ovarian cancer risks depending on the location and type of mutation. Although all regions are associated with increased risk for breast and ovarian cancers among BRCA1/2 carriers, there were specific regions that were associated with even higher cancer risks. …
Permanent link to this article: https://inheritedcancer.net/4nls2015/
Permanent link to this article: https://inheritedcancer.net/1nls2015/
ICARE Newsletter Summer 2015
Ask the Expert
ICARE Newsletter Summer 2015
Ask the Expert
The following question was addressed to Dr. Steven Narod who is a Tier I Canada Research Chair in Breast Cancer and a senior scientist at Women’s College Research Institute in Toronto, Canada. Dr. Narod is a world-leader in the field of breast and ovarian cancer genetics. Over the course of his career, he has profoundly shaped …
Permanent link to this article: https://inheritedcancer.net/5nls2015/
ICARE Newsletter Winter 2015
Highlights of the 2014 National Comprehensive Cancer Network (NCCN) Update
ICARE Newsletter Winter 2015
Highlights of the 2014 National Comprehensive Cancer Network (NCCN) Update
Genetic/Familial High-Risk Assessment: Breast and Ovarian Guidelines For breast cancer screening in BRCA carriers, yearly MRI is recommended starting at age 25; mammograms may be considered in instances where MRI is unavailable or individualized based on earliest age of onset in the family. From age 30-75, annual mammogram and breast MRI is recommended. Above age …
Permanent link to this article: https://inheritedcancer.net/5nlw2015/
ICARE Newsletter Winter 2015
PALB2: A Third Important Gene for Inherited Breast Cancer
ICARE Newsletter Winter 2015
PALB2: A Third Important Gene for Inherited Breast Cancer
Following the publication of an important article in the New England Journal of Medicine (NEJM) in August 2014, germline PALB2 gene mutations were confirmed as the third most important gene for inherited breast cancer, following BRCA1 and BRCA2.1 PALB2 stands for “partner and localizer of BRCA2” and is located on chromosome 16. Studies suggest that PALB2 mutations …
Permanent link to this article: https://inheritedcancer.net/1nlw2015/
ICARE Newsletter Winter 2015
The First PARP-Inhibitor to Be Approved for Clinical Use in BRCA Carriers
ICARE Newsletter Winter 2015
The First PARP-Inhibitor to Be Approved for Clinical Use in BRCA Carriers
More frequently, cancer drugs are being developed to treat tumors based on their molecular make-up. PARP inhibitors are the first class of drugs specifically developed to treat BRCA-related tumors through targeting the DNA repair pathway. The PARP Inhibitors target this pathway and cause cancer cells to die while healthy cells are spared. Although PARP inhibitors …
Permanent link to this article: https://inheritedcancer.net/2nlw2015/
ICARE Newsletter Winter 2015
Ask the Expert
ICARE Newsletter Winter 2015
Ask the Expert
The following question was addressed to Dr. Steven Narod who is a Tier I Canada Research Chair in Breast Cancer and a senior scientist at Women’s College Research Institute in Toronto, Canada. Dr. Narod is a world-leader in the field of breast and ovarian cancer genetics. Over the course of his career, he has profoundly shaped …
Permanent link to this article: https://inheritedcancer.net/3nlw2015/
ICARE Newsletter Summer 2014
Is Breast Cancer Risk Affected by Timing of Oral Contraceptives in BRCA1 Carriers?
ICARE Newsletter Summer 2014
Is Breast Cancer Risk Affected by Timing of Oral Contraceptives in BRCA1 Carriers?
Although oral contraceptives (OC) reduce the risk of ovarian cancer in BRCA carriers,1 it is possible that they may raise breast cancer risk. Thus it is important to understand whether age at OC use is a factor when determining impact on breast cancer risk. To address this question, a recent study (which included data from …
Permanent link to this article: https://inheritedcancer.net/2nls2014/
ICARE Newsletter Summer 2014
What Are Factors That Modify Cancer Risk in BRCA Carriers?
ICARE Newsletter Summer 2014
What Are Factors That Modify Cancer Risk in BRCA Carriers?
Since the discovery of the BRCA genes about two decades ago, a number of studies have reported on factors that may modify cancer risks in those who carry gene mutations. Recently, results of previously published studies were collected through a comprehensive literature review to estimate the overall effects of various risk modifiers in BRCA carriers. …
Permanent link to this article: https://inheritedcancer.net/3nls2014/
ICARE Newsletter Summer 2014
Early Results to Suggest That PSA Screening May Help to Detect Prostate Cancer Early in Men with BRCA Mutations
ICARE Newsletter Summer 2014
Early Results to Suggest That PSA Screening May Help to Detect Prostate Cancer Early in Men with BRCA Mutations
Over the last few years, there have been a number of studies to suggest that men with BRCA mutations, particularly BRCA2, have a higher risk of developing aggressive prostate cancer. It remains uncertain whether these men might benefit from screening through the prostate-specific antigen (PSA) test. Within the last few years, PSA screening guidelines in …
Permanent link to this article: https://inheritedcancer.net/1nls2014/
ICARE Newsletter Summer 2014
Ask the Expert
ICARE Newsletter Summer 2014
Ask the Expert
The following question was addressed by Dr. Steven Narod who is a Tier I Canada Research Chair in Breast Cancer and a senior scientist at Women’s College Research Institute in Toronto, Canada. Dr. Narod is a world-leader in the field of breast and ovarian cancer genetics. Over the course of his career, he has profoundly shaped …
Permanent link to this article: https://inheritedcancer.net/5nls2014/
ICARE Newsletter Summer 2014
New Study to Suggest Benefits of Oophorectomy in BRCA Mutation Carriers
ICARE Newsletter Summer 2014
New Study to Suggest Benefits of Oophorectomy in BRCA Mutation Carriers
A recent study published in the Journal of Clinical Oncology reported that prophylactic oophorectomy resulted in reducing the risk of ovarian cancer by 80%, and reduced all-cause mortality by 77%.1 The authors reported results on almost 5800 women, of whom 186 developed either ovarian, fallopian tube or peritoneal cancer. Women who had bilateral oophorectomy had …
Permanent link to this article: https://inheritedcancer.net/4nls2014/
ICARE Newsletter Winter 2014
Tamoxifen May Reduce Contralateral Breast Cancer Risk in BRCA Carriers
ICARE Newsletter Winter 2014
Tamoxifen May Reduce Contralateral Breast Cancer Risk in BRCA Carriers
There have been suggestions that Tamoxifen may reduce risks for contralateral breast cancer (i.e., breast cancer in the other breast) in BRCA carriers if taken after the initial breast cancer diagnosis, based mainly on retrospective studies. Only one prospective study has looked at this question, and showed that Tamoxifen may be useful in BRCA2, but …
Permanent link to this article: https://inheritedcancer.net/2nlw2014/
ICARE Newsletter Winter 2014
Ask the Expert
ICARE Newsletter Winter 2014
Ask the Expert
The following question was addressed by Dr. Laronga who is a breast surgeon based at the Moffitt Cancer Center: Q. What are the risks of breast cancer after ovarian cancer in BRCA carriers? What risk management options are recommended? A. BRCA carriers remain at a higher risk of breast cancer, even after having ovarian cancer; …
Permanent link to this article: https://inheritedcancer.net/4nlw2014/
ICARE Newsletter Winter 2014
Contralateral Mastectomy May Improve Survival in BRCA Mutation Carriers
ICARE Newsletter Winter 2014
Contralateral Mastectomy May Improve Survival in BRCA Mutation Carriers
It has been established that women who carry a germline BRCA mutation face breast cancer risks of 60-70% in their lifetime. After an initial breast cancer diagnosis, these women face a high risk for contralateral breast cancer. Some women with BRCA mutations move forward with contralateral mastectomy when they develop their first breast cancer diagnosis (as …
Permanent link to this article: https://inheritedcancer.net/1nlw2014/
ICARE Newsletter Summer 2013
Sharing BRCA Test Results with Adolescent and Young Adult Children—What Does the Latest Research Show?
ICARE Newsletter Summer 2013
Sharing BRCA Test Results with Adolescent and Young Adult Children—What Does the Latest Research Show?
While there are specific recommendations against BRCA testing for minors,1 guidelines are less clear about whether parents should share their own test results with their children. Because there are no recommended surveillance or risk reduction options prior to age 25 for known BRCA mutation carriers, there has been debate about balancing the benefits of sharing …
Permanent link to this article: https://inheritedcancer.net/2nls2013/
ICARE Newsletter Summer 2013
US Preventive Services Task Force (USPSTF) Guidelines for Inherited Breast and Ovarian Cancer and Implications to the Affordable Care Act (ACA)
ICARE Newsletter Summer 2013
US Preventive Services Task Force (USPSTF) Guidelines for Inherited Breast and Ovarian Cancer and Implications to the Affordable Care Act (ACA)
Recently, the USPSTF released updated draft guidelines in April 2013 (from those previously published in 2005) for inherited breast and ovarian cancer due to germline BRCA1 and BRCA2 gene mutations.1 USPSTF is comprised of primary care providers who review the available literature and issue guidelines about risk assessment, testing and management based on available evidence. …
Permanent link to this article: https://inheritedcancer.net/4nls2013/
ICARE Newsletter Summer 2013
Male BRCA Carriers Have Poorer Outcomes from Prostate Cancer
ICARE Newsletter Summer 2013
Male BRCA Carriers Have Poorer Outcomes from Prostate Cancer
Over the last few years, a number of studies have suggested that men with germline BRCA mutations (especially BRCA2) have poorer outcomes when they develop prostate cancer. In fact, a recent study of 2019 patients with prostate cancer, including 18 BRCA1 carriers, 61 BRCA2 carriers, and 1940 noncarriers indicated that germline mutations were more frequently …
Permanent link to this article: https://inheritedcancer.net/3nls2013/
ICARE Newsletter Summer 2013
Oophorectomy Following Menopause
ICARE Newsletter Summer 2013
Oophorectomy Following Menopause
Prior studies have indicated that removal of the ovaries and fallopian tubes reduces the ovarian cancer risk by ~80% and breast cancer risk by ~50%, particularly when performed pre-menopausally. However a recent case control study of 2854 pairs of women with a BRCA1 or BRCA2 mutation with or without breast cancer showed that the risk …
Permanent link to this article: https://inheritedcancer.net/5nls2013/
ICARE Newsletter Summer 2013
BRCA Testing: Supreme Court Update
ICARE Newsletter Summer 2013
BRCA Testing: Supreme Court Update
In a landmark decision regarding the patenting of human genes on Thursday June 13, 2013, the Supreme Court of the United States unanimously ruled that human genes may not be patented. The case specifically concerned the BRCA1 and BRCA2 gene patents, held by the Utah-based company, Myriad Genetics. In the ruling, Justice Clarence Thomas wrote for the …
Permanent link to this article: https://inheritedcancer.net/1nls2013/
ICARE Newsletter Summer 2013
Ask the Expert
ICARE Newsletter Summer 2013
Ask the Expert
The following question was addressed by Dr. Pal, who is a Clinical Geneticist based at the Moffitt Cancer Center: Q. Does exposure to radiation increase breast cancer risk in BRCA mutation carriers? A. A number of studies have been conducted to evaluate whether BRCA mutation carriers may be more prone to radiation-induced breast cancer than …
Permanent link to this article: https://inheritedcancer.net/6nls2013/
ICARE Newsletter Winter 2013
Ask the Expert
ICARE Newsletter Winter 2013
Ask the Expert
The following question was addressed by Dr. Lora Thompson, a Clinical Psychologist at the Moffitt Cancer Center: Q. How do I talk to family members about my genetic test results? A. The ability to share risk information with family members is a common reason why many individuals undergo genetic testing. Family members may feel appreciative …
Permanent link to this article: https://inheritedcancer.net/4nlw2013/
ICARE Newsletter Winter 2013
Points to Consider Regarding Bilateral Salpingectomy as a Risk Reduction Procedure for Ovarian Cancer
ICARE Newsletter Winter 2013
Points to Consider Regarding Bilateral Salpingectomy as a Risk Reduction Procedure for Ovarian Cancer
Over the last few years, there has been evidence to suggest that a substantial proportion of ovarian cancer may start in the fallopian tubes, although some cancer clearly arises in the ovary. As a result, removal of both fallopian tubes (called ‘bilateral salpingectomy’) has been suggested as an interim procedure to reduce risk in BRCA …
Permanent link to this article: https://inheritedcancer.net/2nlw2013/
ICARE Newsletter Winter 2013
The Selection of Chemotherapy in BRCA Patients with Pancreatic Cancer
ICARE Newsletter Winter 2013
The Selection of Chemotherapy in BRCA Patients with Pancreatic Cancer
Some evidence suggests that individuals with BRCA mutations who develop pancreatic cancer may benefit from specific chemotherapy regimens. In a recent review of this topic, Kim et al reported on a study of 5 patients with BRCA mutations (4 BRCA2 and 1 BRCA1) who were treated with a platinum-based chemotherapy regimen.1 Of these patients, 3 …
Permanent link to this article: https://inheritedcancer.net/1nlw2013/
ICARE Newsletter Summer 2012
Ask the Expert: What are the risks of hormone replacement therapy (HRT) on breast cancer risk in women with BRCA mutations?
ICARE Newsletter Summer 2012
Ask the Expert: What are the risks of hormone replacement therapy (HRT) on breast cancer risk in women with BRCA mutations?
The following questions were addressed by Drs. Pal and Lancaster at the Moffitt Cancer Center: Q. What are the risks of hormone replacement therapy (HRT) on breast cancer risk in women with BRCA mutations? A. Concern about HRT in BRCA carriers is its potential to raise the risk of breast cancer, as seen in the …
Permanent link to this article: https://inheritedcancer.net/4nls2012/
ICARE Newsletter Summer 2012
New Study Suggests Breast Cancer Risk for Non-Carriers of Family-Specific BRCA Mutations Is Not Increased
ICARE Newsletter Summer 2012
New Study Suggests Breast Cancer Risk for Non-Carriers of Family-Specific BRCA Mutations Is Not Increased
Data from a population-based study was recently reported to estimate breast cancer risk among family members who tested negative for a known BRCA1/2 family mutation (i.e., non-carriers). The study included 3047 women diagnosed with breast cancer. Results from the study indicated there was no increased risk for breast cancer in non-carriers as compared to family …
Permanent link to this article: https://inheritedcancer.net/3nls2012/
ICARE Newsletter Summer 2012
Emerging Cancer Panels for Testing Patients for Inherited Cancer Predisposition
ICARE Newsletter Summer 2012
Emerging Cancer Panels for Testing Patients for Inherited Cancer Predisposition
Genetic testing for inherited cancer predisposition is typically performed by testing for one condition at a time. However, with the tremendous advances in genetic testing technologies over the last few years, the cost of testing has plummeted. To put this into perspective, the first human genome cost 2-3 billion dollars to sequence and took over …
Permanent link to this article: https://inheritedcancer.net/1nls2012/
ICARE Newsletter Summer 2012
Ask the Expert: What are the recommendations for screening following prophylactic surgeries?
ICARE Newsletter Summer 2012
Ask the Expert: What are the recommendations for screening following prophylactic surgeries?
The following questions were addressed by Drs. Pal and Lancaster at the Moffitt Cancer Center: Q. What are the recommendations for screening following prophylactic surgeries? A. In BRCA mutation carriers, bilateral prophylactic mastectomy reduces the risk of breast cancer by over 90%. It essentially lowers the risk of breast cancer to below that of the …
Permanent link to this article: https://inheritedcancer.net/5nls2012/
ICARE Newsletter Summer 2012
Prostate Cancer Screening Recommendations for Men with BRCA Mutations
ICARE Newsletter Summer 2012
Prostate Cancer Screening Recommendations for Men with BRCA Mutations
Over the last few years, there have been several studies that suggest that men with BRCA mutations are at a higher risk for developing and dying from aggressive prostate cancer. It is possible that PSA testing may be of benefit in men with BRCA mutations. However, until the utility of PSA is determined in these …
Permanent link to this article: https://inheritedcancer.net/2nls2012/