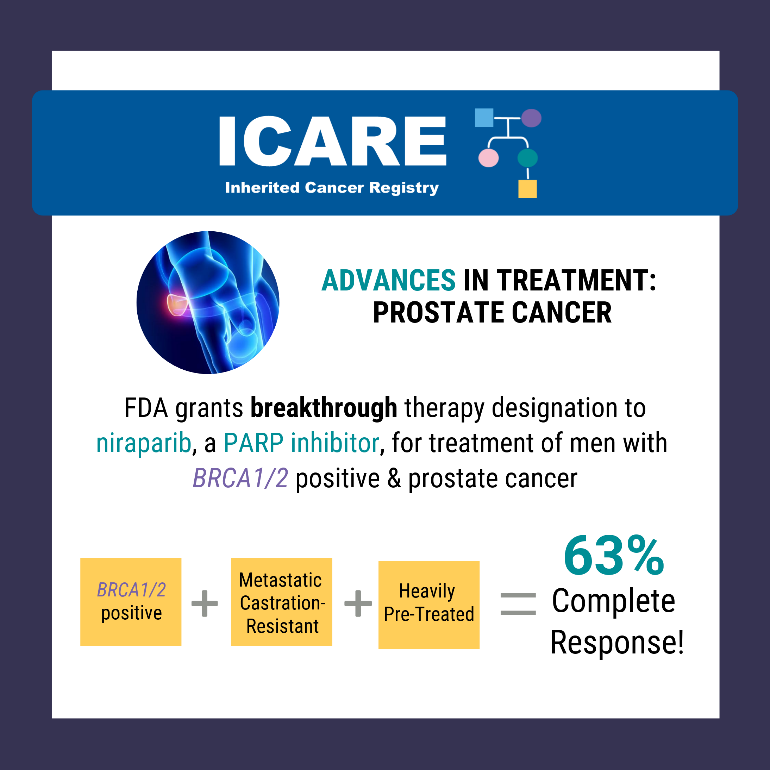
 The FDA granted breakthrough therapy designation to niraparib (a PARP inhibitor) for the treatment of men with BRCA1/2 positive, metastatic castration-resistant, and heavily pre-treated prostate cancer.
The FDA granted breakthrough therapy designation to niraparib (a PARP inhibitor) for the treatment of men with BRCA1/2 positive, metastatic castration-resistant, and heavily pre-treated prostate cancer.
Results from a recent study show a 63% complete response rate in men with BRCA1/2 positive, metastatic castration-resistant prostate cancer treated with niraparib compared to 17% in the non- BRCA group.
Previously, niraparib was approved for ovarian cancer. This treatment can help address lack of treatment options for men with BRCA1/2 positive, metastatic castration-resistant prostate cancer.
Check out the articles at https://www.cancernetwork.com/fda/fda-grants-breakthrough-therapy-designation-niraparib-mcrpc and https://www.healio.com/hematology-oncology/prostate-cancer/news/online/%7B22878907-82bd-408e-b99f-b51c50d8c467%7D/fda-grants-breakthrough-therapy-designation-to-zejula-for-prostate-cancer-subtype.
