In January 2024, the American Society of Clinical Oncology (ASCO) in conjunction with the Society of Surgical Oncology released new guidelines for germline testing in patients with breast cancer, which include the following: For a full list of recommendations in this guideline, the article is available at: https://ascopubs.org/doi/10.1200/JCO.23.02225 Bedrosian, et al. J Clin Oncol. 2024;42(5):584-604. PMID:38175972. Social …
Tag: Guideline/Policy Updates
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2024-new-guidelines-released-through-asco-society-of-oncology-germline-testing-in-patients-with-breast-center/
ICARE Newsletter Spring 2024
National Comprehensive Cancer Network (NCCN) Guideline Updates
ICARE Newsletter Spring 2024
National Comprehensive Cancer Network (NCCN) Guideline Updates
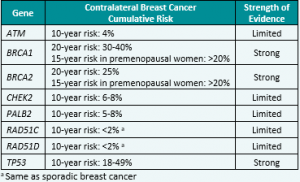
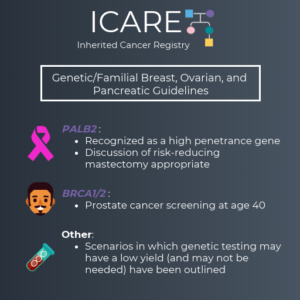
Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic Cancer – Released February 12th, 2024 (V3.2024) Check out the full guidelines by creating a FREE account at www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf Contralateral breast cancer risks in these updated guidelines: Expanded guidance about gynecologic cancers in BRCA1/2 carriers: Some highlights related to HRT include: Genetic/Familial High-Risk Assessment: Colorectal Cancer – Released …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2024-national-comprehensive-cancer-network-nccn-guideline-updates/
ICARE Featured Video March 2024
NCCN Genetic/Familial Guideline Updates: Breast, Ovarian, Pancreatic (V3.2024)
ICARE Featured Video March 2024
NCCN Genetic/Familial Guideline Updates: Breast, Ovarian, Pancreatic (V3.2024)
Below is a featured video from the March 2024 case conference, during which Tuya Pal, MD, FACMG from Vanderbilt University Medical Center presents on recent updated to the National Comprehensive Cancer Network (NCCN) Genetic/Familial Breast, Ovarian, and Pancreatic cancer guidelines.
Permanent link to this article: https://inheritedcancer.net/video30724_2/
ICARE Newsletter Fall 2023
Recommendations for Inherited Ovarian Cancer Genes: United Kingdom (UK)
ICARE Newsletter Fall 2023
Recommendations for Inherited Ovarian Cancer Genes: United Kingdom (UK)
Through consensus, management recommendations were developed in the UK for females with pathogenic variants inthe following inherited ovarian cancer genes: RAD51C, RAD51D, BRIP1, and PALB2. Hanson, et al. J Med Genet. 2023;60(5):417-429. PMID: 36411032. Social media post September 26th, 2023.Available at https://tinyurl.com/post9262023.
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2023-recommendations-for-inherited-ovarian-cancer-genes-united-kingdom-uk/
ICARE Newsletter Fall 2023
Mammograms: New U.S. Preventive Services Task Force (USPSTF) Recommendations
ICARE Newsletter Fall 2023
Mammograms: New U.S. Preventive Services Task Force (USPSTF) Recommendations
On May 9th, 2023, the USPSTF published a new draft recommendation for all cisgender women and those assigned female sex at birth to do mammograms from ages 40 to 74, every two years, for those at average (population) risk forbreast cancer. This change in recommendation is due to recent troubling trends, including an increase in …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2023-mammograms-new-u-s-preventive-services-task-force-uspstf-recommendations/
ICARE Featured Video September 2023
NCCN Genetic/Familial Guideline Updates: Breast, Ovarian, Pancreatic (V1.2024)
ICARE Featured Video September 2023
NCCN Genetic/Familial Guideline Updates: Breast, Ovarian, Pancreatic (V1.2024)
Below is a featured video from the September 2023 case conference, during which Tuya Pal, MD, FACMG from Vanderbilt University Medical Center presents on recent updated to the National Comprehensive Cancer Network (NCCN) Genetic/Familial Breast, Ovarian, and Pancreatic cancer guidelines. Please visit our YouTube channel to watch the full September conference, including valuable discussion about …
Permanent link to this article: https://inheritedcancer.net/video91423/
ICARE Featured Video October 2022
NCCN Genetic/Familial Guideline Updates (V1.2023 & V.1.2022)
ICARE Featured Video October 2022
NCCN Genetic/Familial Guideline Updates (V1.2023 & V.1.2022)
Below you may watch a featured video from the October 2022 Genetics Case Conference in which ICARE Principal Investigator, Tuya Pal, MD, discusses recent updates to the National Comprehensive Cancer Network (NCCN) guidelines.
Permanent link to this article: https://inheritedcancer.net/video100622/
ICARE Social Media Post September 2021
New NCCN Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic Guidelines (Released August 11th)
ICARE Social Media Post September 2021
New NCCN Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic Guidelines (Released August 11th)
The National Comprehensive Cancer Network (NCCN) released new guidelines on August 11th for Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. You can check out the full guidelines by creating a FREE account at: https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf
Permanent link to this article: https://inheritedcancer.net/post91021/
Permanent link to this article: https://inheritedcancer.net/post83121/
ICARE Social Media Post August 2021
New York Times PALB2 Article
ICARE Social Media Post August 2021
New York Times PALB2 Article
A New York Times article just published focused on the importance of PALB2 as a breast cancer gene (https://www.nytimes.com/…/breast-cancer-palb2-brca.html), which referenced our recent article focused on managing PALB2 carriers sponsored by ACMG – American College of Medical Genetics and Genomics, and developed through a worldwide collaboration (including: Marc Tischkowicz, Judith Balmana, Will Foulkes, Paul James, …
Permanent link to this article: https://inheritedcancer.net/post81921/
ICARE Social Media Post August 2021
ASCO Guideline Update: Olaparib for Breast Cancer
ICARE Social Media Post August 2021
ASCO Guideline Update: Olaparib for Breast Cancer
For additional information, read the updated American Society of Clinical Oncology (ASCO) recommendation (released June 15th, 2021) at the following link: https://www.asco.org/practice-patients/guidelines/breast-cancer?intcmp=ws_ascoorg_gdlns_hereditarybreastcancer_site_pressrelease_061621____#/143725
Permanent link to this article: https://inheritedcancer.net/post81021/
ICARE Social Media Post May 2021
USPSTF Earlier Colorectal Cancer Screening At Age 45
ICARE Social Media Post May 2021
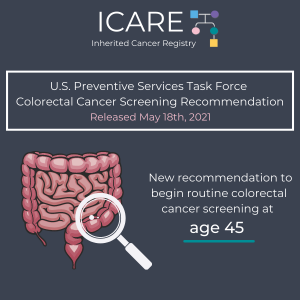
USPSTF Earlier Colorectal Cancer Screening At Age 45
The United States Preventive Services Task Force (USPSTF) now recommends routine colorectal cancer screening begin at age 45 (lowered from the previous recommendation to start screening at age 50) due to an increasing number of colorectal cancer cases in younger adults. For more information, check out the full JAMA article at: https://jamanetwork.com/journals/jama/fullarticle/2779985
Permanent link to this article: https://inheritedcancer.net/post52121/
ICARE Social Media Post May 2021
ACMG Gene List
ICARE Social Media Post May 2021
ACMG Gene List
The American College of Medical Genetics and Genomics (ACMG) recently released the highly anticipated recommended minimum gene list for reporting secondary findings (SF) in clinical exome and genome sequencing. ACMG SF v3.0 added 3 new cancer genes (𝘗𝘈𝘓𝘉2, 𝘔𝘈𝘟, and 𝘛𝘔𝘌𝘔127) to bring the total number of inherited cancer genes to 28. For more information …
Permanent link to this article: https://inheritedcancer.net/post52121_2/
ICARE Social Media Post May 2021
ACMG Clinical Practice Resource on PALB2
ICARE Social Media Post May 2021
ACMG Clinical Practice Resource on PALB2
Check out the newly released ACMG Clinical Practice Resource on 𝗣𝗔𝗟𝗕𝟮 developed through a group of worldwide experts!https://www.acmg.net/PDFLibrary/41436_2021_1151_OnlinePDF.pdf Read the ACMG press release at: https://www.acmg.net/PDFLibrary/Global%20Team%20of%20Cancer%20Genetic%20Specialists%20release%20final%20template%205%206.final.pdf
Permanent link to this article: https://inheritedcancer.net/post51121/
ICARE Social Media Post April 2021
Reducing Hereditary Cancer Act
ICARE Social Media Post April 2021
Reducing Hereditary Cancer Act
The 𝙍𝙚𝙙𝙪𝙘𝙞𝙣𝙜 𝙃𝙚𝙧𝙚𝙙𝙞𝙩𝙖𝙧𝙮 𝘾𝙖𝙣𝙘𝙚𝙧 𝘼𝙘𝙩 is being proposed to ensure Medicare beneficiaries have access to inherited cancer genetic testing, increased screening, and risk-reducing procedures, when medically necessary & appropriate. Under current Medicare guidelines, only those with “signs, symptoms, complaints, or personal histories of disease” meet the criteria for medical services coverage. Genetic testing is only …
Permanent link to this article: https://inheritedcancer.net/post43021/
Permanent link to this article: https://inheritedcancer.net/video31121/
Permanent link to this article: https://inheritedcancer.net/1nlw2021/
ICARE Newsletter Winter 2021
Inherited Cancer Treatment: Updates and Relevant Policies
ICARE Newsletter Winter 2021
Inherited Cancer Treatment: Updates and Relevant Policies
Over the last several months, the American Society of Clinical Oncology published a number of guidelines related to the use of PARP inhibitors among those with BRCA-associated cancers, including guidelines focused on ovarian cancer,1 metastatic pancreatic cancer,2 and breast cancer.3 Additionally, costs of drugs also have great potential to influence policy, highlighting the importance of …
Permanent link to this article: https://inheritedcancer.net/7nlw2021/
ICARE Social Media Post February 2021
ICARE Winter 2021 Newsletter
ICARE Social Media Post February 2021
ICARE Winter 2021 Newsletter
The ICARE Winter 2021 Newsletter is now available! Check out this latest edition for recent research and clinical updates, a Q&A with a genomics expert, and an inspiring community spotlight piece. You can read the newsletter by visiting: https://inheritedcancer.net/wp-content/uploads/ICARE-2021-Winter-Newsletter.pdf. Please feel free to share with family members, friends, and/or your healthcare providers.
Permanent link to this article: https://inheritedcancer.net/post20921/
ICARE Featured Video December 2020
NCCN Genetic/Familial Breast, Ovarian, and Pancreatic Guidelines
ICARE Featured Video December 2020
NCCN Genetic/Familial Breast, Ovarian, and Pancreatic Guidelines
Below you may watch a featured video from the December 2020 Genetics Case Conference, which outlined updates to the National Comprehensive Cancer Network (NCCN) guidelines. Check out the full guidelines by creating a FREE account at: https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf
Permanent link to this article: https://inheritedcancer.net/video121020/
Permanent link to this article: https://inheritedcancer.net/post120820/
ICARE Social Media Post November 2020
ASCO Guideline Updates: Breast Cancer
ICARE Social Media Post November 2020
ASCO Guideline Updates: Breast Cancer
The American Society of Clinical Oncology (ASCO) published updated guidelines for the management of hereditary breast cancer for the following gene carriers: 𝘽𝙍𝘾𝘼1/2 • Consider breast-conserving therapy • Consider nipple-sparing mastectomy, if medically appropriate • Advanced breast cancer: ⫸ PARP inhibitors (olaparib, talazoparib) preferred over non-platinum single agent chemotherapy ⫸ Platinum agents are recommended …
Permanent link to this article: https://inheritedcancer.net/post112020/
Permanent link to this article: https://inheritedcancer.net/post111720/
ICARE Social Media Post October 2020
New ASCO Guidelines On Use Of PARP Inhibitors To Manage Ovarian Cancer
ICARE Social Media Post October 2020
New ASCO Guidelines On Use Of PARP Inhibitors To Manage Ovarian Cancer
New guidelines for the use of PARP inhibitors to treat ovarian cancer among those with BRCA1 or BRCA2 mutations were published through the American Society of Clinical Oncology (ASCO) to guide providers about the role of this class of drugs in the management of this type of cancer. Link to the guidelines are available at: …
Permanent link to this article: https://inheritedcancer.net/post101320/
Permanent link to this article: https://inheritedcancer.net/video100820/
ICARE Social Media Post September 2020
ICARE Summer 2020 Newsletter
ICARE Social Media Post September 2020
ICARE Summer 2020 Newsletter
The ICARE Summer 2020 Newsletter is now available! Check out this latest edition for recent research and clinical updates as well as a Q&A with a nationally renowned clinical geneticist from Vanderbilt-Ingram Cancer Center. You can read the newsletter by visiting: https://inheritedcancer.net/wp-content/uploads/ICARE-2020-Summer-Newsletter.pdf Please feel free to share with family members, friends, and/or your …
Permanent link to this article: https://inheritedcancer.net/post91620/
ICARE Social Media Post September 2020
NCCN Genetic Testing Criteria Updates by Cancer
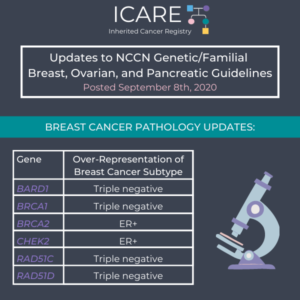
ICARE Social Media Post September 2020
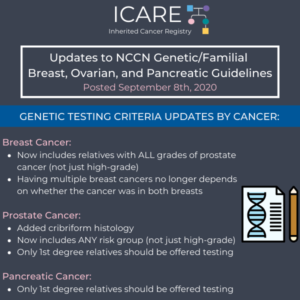
NCCN Genetic Testing Criteria Updates by Cancer
The National Comprehensive Cancer Network (NCCN) released new guidelines on September 8th, 2020, which included updates to genetic testing criteria by cancer type as follows: Breast Cancer: Broadened to include relatives with ALL grades of prostate cancer (not just high-grade) Now having multiple breast cancer diagnoses is NOT dependent on whether the diagnoses were …
Permanent link to this article: https://inheritedcancer.net/post91520/
Permanent link to this article: https://inheritedcancer.net/post91420/
ICARE Social Media Post September 2020
NCCN Genetic Testing Choice Updates
ICARE Social Media Post September 2020
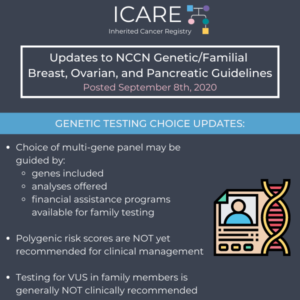
NCCN Genetic Testing Choice Updates
The National Comprehensive Cancer Network (NCCN) released new guidelines on September 8th, 2020, which included updates to genetic testing choices and considerations as follows: Choice of multi-gene panel may be guided by genes included, analyses offered, and financial assistance programs available for family testing Significant limitations in interpretations of polygenic risk scores: NOT recommended for …
Permanent link to this article: https://inheritedcancer.net/post91120/
Permanent link to this article: https://inheritedcancer.net/post91020/
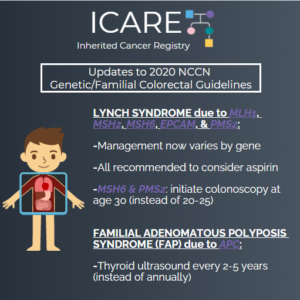
ICARE Social Media Post July 2020
Updates to 2020 NCCN Genetic/Familial Colorectal Guidelines
ICARE Social Media Post July 2020
Updates to 2020 NCCN Genetic/Familial Colorectal Guidelines
The National Comprehensive Cancer Network (NCCN) released new guidelines for 2020 on July 21, 2020. The big changes included refining some of the risks for genes involved in Lynch Syndrome, and providing specific guidance about cancer screening that may slightly differ by gene. You can check out the full guidelines by creating a FREE account …
Permanent link to this article: https://inheritedcancer.net/post72420/
ICARE Social Media Post April 2020
ASCO Guideline: Genetic Testing for Ovarian Cancer
ICARE Social Media Post April 2020
ASCO Guideline: Genetic Testing for Ovarian Cancer
The American Society of Clinical Oncology (ASCO) recently published a guideline reinforcing the longstanding recommendation that all women diagnosed with epithelial ovarian cancer (EOC) be offered genetic testing for hereditary ovarian cancer genes. Many of these women (>15%) have an inherited mutation, most commonly BRCA1 or BRCA2. Identifying BRCA1/2 mutations may help guide cancer treatment. …
Permanent link to this article: https://inheritedcancer.net/post41720/
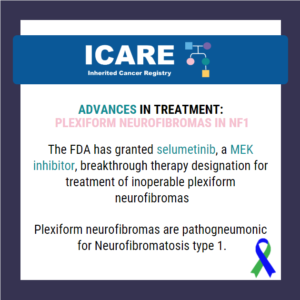
ICARE Social Media Post February 2020
Advances in Treatment: Plexiform Neurofibromas in NF1
ICARE Social Media Post February 2020
Advances in Treatment: Plexiform Neurofibromas in NF1
The FDA has granted a breakthrough therapy designation to selumetinib, a MEK inhibitor, for treatment of inoperable plexiform neurofibromas. These types of neurofibromas are almost exclusively seen in individuals with neurofibromatosis type 1 (NF1). These plexiform neurofibromas are benign tumors on the nerve sheaths and can develop anywhere in the body. These tumors typically cause …
Permanent link to this article: https://inheritedcancer.net/post22820/
ICARE Newsletter Winter 2020
Treatment Advances Among Those with Inherited Prostate Cancer Predisposition
ICARE Newsletter Winter 2020
Treatment Advances Among Those with Inherited Prostate Cancer Predisposition
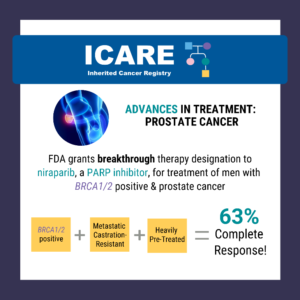
A recent study reported a high complete response rate among men with a BRCA1/2 mutation with metastatic, castration-resistant prostate cancer who were treated with niraparib (a PARP inhibitor) of 63% compared to 17% in the non-BRCA1/2 group.1 Based on this data, the Federal Drug Administration (FDA) granted breakthrough therapy designation to niraparib on October 3, …
Permanent link to this article: https://inheritedcancer.net/3nlw2020/
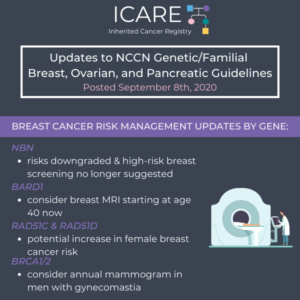
ICARE Newsletter Winter 2020
Updates to National Comprehensive Cancer Network (NCCN) Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic
ICARE Newsletter Winter 2020
Updates to National Comprehensive Cancer Network (NCCN) Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic
There were significant updates and restructuring of the guidelines, with some highlights included below: Substantial reorganization of the guidelines as follows: Now organized by organ site, rather than primarily by certain high penetrance genes Focused efforts to simplify genetic testing criteria Only one flow diagram included, to outline the ‘genetic testing process’ Following scenarios now …
Permanent link to this article: https://inheritedcancer.net/1nlw2020/
ICARE Newsletter Winter 2020
Updated Pancreatic Cancer Screening Guidelines through CAPS Consortium
ICARE Newsletter Winter 2020
Updated Pancreatic Cancer Screening Guidelines through CAPS Consortium
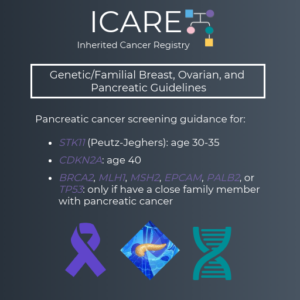
The International Cancer of the Pancreas Screening (CAPS) Consortium recently published updated recommendations about pancreatic cancer screening through MRI/magnetic retrograde cholangiopancreatography (MRCP) and/or an endoscopic ultrasound (EUS).1 Specifically, these guidelines now recommend that individuals with a CDKN2A or STK11 mutation begin screening at age 40. Screening for individuals with a BRCA1/2, ATM, PALB2, MLH1, or …
Permanent link to this article: https://inheritedcancer.net/4nlw2020/
ICARE Social Media Post February 2020
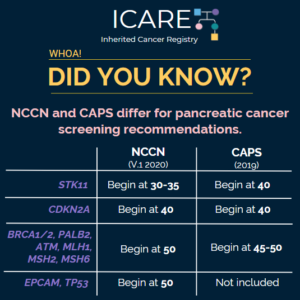
Differences in Pancreatic Cancer Screening Recommendations from the National Comprehensive Cancer Network (NCCN) and the International Cancer of the Pancreas Screening (CAPS) Consortium
ICARE Social Media Post February 2020
Differences in Pancreatic Cancer Screening Recommendations from the National Comprehensive Cancer Network (NCCN) and the International Cancer of the Pancreas Screening (CAPS) Consortium
The National Comprehensive Cancer Network (NCCN) and the International Cancer of the Pancreas Screening (CAPS) Consortium recently updated pancreatic cancer screening recommendations. However, there are some differences between these recommendations. Specifically, screening with annual MRI/magnetic retrograde cholangiopancreatography (MRCP) and/or endoscopic ultrasound (EUS) is recommended as follows for NCCN versus CAPS: STK11 regardless of family history: …
Permanent link to this article: https://inheritedcancer.net/post2620/
ICARE Social Media Post February 2020
Updated Pancreatic Cancer Screening Guidelines through the International Cancer of the Pancreas Screening (CAPS) Consortium
ICARE Social Media Post February 2020
Updated Pancreatic Cancer Screening Guidelines through the International Cancer of the Pancreas Screening (CAPS) Consortium
The International Cancer of the Pancreas Screening (CAPS) Consortium recently published updated pancreatic cancer screening recommendations. The recommendations include: Screening with MRI/magnetic retrograde cholangiopancreaography (MRCP) and/or endoscopic ultrasound (EUS) The screening was recommended for the following individuals: CDKN2A and STK11 mutation carriers starting at age 40 BRCA1/2, ATM, PALB2, MLH1, and MSH2 mutation carriers (if …
Permanent link to this article: https://inheritedcancer.net/post2420/
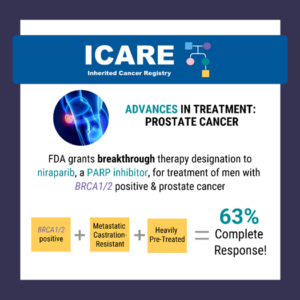
ICARE Social Media Post December 2019
Evaluation of PARP Inhibitors in BRCA-Associated Prostate Cancer
ICARE Social Media Post December 2019
Evaluation of PARP Inhibitors in BRCA-Associated Prostate Cancer
The FDA granted breakthrough therapy designation to niraparib (a PARP inhibitor) for the treatment of men with BRCA1/2 positive, metastatic castration-resistant, and heavily pre-treated prostate cancer. Results from a recent study show a 63% complete response rate in men with BRCA1/2 positive, metastatic castration-resistant prostate cancer treated with niraparib compared to 17% in the non- …
Permanent link to this article: https://inheritedcancer.net/post122019/
ICARE Social Media Post December 2019
Updates to National Comprehensive Cancer Network (NCCN) Genetic/Familial Breast, Ovarian, and Pancreatic Guidelines (V1.2020)
ICARE Social Media Post December 2019
Updates to National Comprehensive Cancer Network (NCCN) Genetic/Familial Breast, Ovarian, and Pancreatic Guidelines (V1.2020)
We are excited to share the latest version of the NCCN Genetic/Familial Breast, Ovarian and Pancreatic Guidelines (V1.2020), which were just updated. Some of the changes made include: PALB2 was added as a high penetrance gene (similar to BRCA1, BRCA2, CDH1, PTEN and TP53) It is appropriate to consider risk reducing mastectomy for cancer risk management …
Permanent link to this article: https://inheritedcancer.net/post12419/
ICARE Newsletter Summer 2019
Updates to National Comprehensive Cancer Network (NCCN) Genetic/Familial High-Risk Assessment: Colorectal Guidelines
ICARE Newsletter Summer 2019
Updates to National Comprehensive Cancer Network (NCCN) Genetic/Familial High-Risk Assessment: Colorectal Guidelines
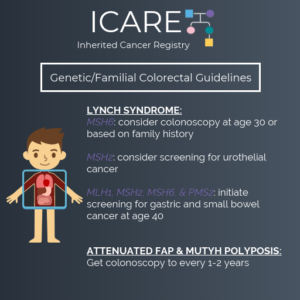
(Version 1.2019, posted July 3, 2019) For Individuals with Lynch Syndrome: The cancer risk table was updated: Addition of new cancer risks by specific genes: breast and bladder cancers Updates of cancer risks by specific genes: ovarian, prostate, gastric, pancreatic, urothelial, small bowel, and brain/CNS cancers Removal of reference to sebaceous neoplasms Recommendations for cancer …
Permanent link to this article: https://inheritedcancer.net/1nls2019/
ICARE Newsletter Summer 2019
Expansion of Criteria for BRCA1/2 Testing through the USPSTF
ICARE Newsletter Summer 2019
Expansion of Criteria for BRCA1/2 Testing through the USPSTF
The U.S. Preventive Services Task Force (USPSTF) came out with new genetic testing guidelines for the BRCA1/2 genes, which has garnered substantial media attention. This task force consists of a team of primary care and preventive medicine healthcare experts to lower the chance of a conflict of interest (which is also the reason that subspecialty …
Permanent link to this article: https://inheritedcancer.net/5nls2019/
Permanent link to this article: https://inheritedcancer.net/1nls2018/
ICARE Newsletter Summer 2018
Updates to NCCN Genetic/Familial High-Risk Assessment: Colorectal Guidelines
ICARE Newsletter Summer 2018
Updates to NCCN Genetic/Familial High-Risk Assessment: Colorectal Guidelines
For Individuals with Lynch Syndrome: Surveillance for gastric and small bowel cancer now indicates there is no clear data to support this, but surveillance can be performed every 3-5 years starting at age 40 Lack of evidence to make a recommendation for pancreatic or prostate cancer screening, beyond those already recommended through other NCCN Guideline …
Permanent link to this article: https://inheritedcancer.net/2nls2018/
ICARE Newsletter Winter 2018
Updates to NCCN Genetic/Familial High-Risk Assessment: Breast and Ovarian Guidelines
ICARE Newsletter Winter 2018
Updates to NCCN Genetic/Familial High-Risk Assessment: Breast and Ovarian Guidelines
(Version 1.2018, posted Oct. 3, 2017) Metastatic prostate cancer was added as an indication for evaluation and testing for the BRCA1 and BRCA2 genes Among BRCA1, BRCA2, TP53 and PTEN carriers, women between ages 25-29 may consider having an annual mammogram with consideration of tomosynthesis if a breast MRI is not available. Among female BRCA2 …
Permanent link to this article: https://inheritedcancer.net/1nlw2018/
ICARE Newsletter Winter 2018
FDA Approval of PARP Inhibitor (Lynparza) for Treatment of Advanced Breast Cancer
ICARE Newsletter Winter 2018
FDA Approval of PARP Inhibitor (Lynparza) for Treatment of Advanced Breast Cancer
On January 12, 2018, the FDA approved the first PARP Inhibitor (Lynparza) for treatment in patients with advanced breast cancer due to inherited BRCA mutations.1 This drug is already approved for certain BRCA carriers for advanced ovarian cancer. PARP inhibitors were originally developed to target the specific pathway through which cancer develops among those with …
Permanent link to this article: https://inheritedcancer.net/6nlw2018/
ICARE Newsletter Summer 2017
Emerging FDA Approvals of Immunotherapy Among Patients With Metastatic MSI-H Cancers
ICARE Newsletter Summer 2017
Emerging FDA Approvals of Immunotherapy Among Patients With Metastatic MSI-H Cancers
Over the last few years, immunotherapy has emerged as an exciting new class of drugs. As early as 2015, immunotherapy through PD-1 Inhibitors among patients with MSI-H colorectal cancers was shown to be of potential benefit.1 As many individuals with Lynch Syndrome have cancers that are MSI-H and mismatch repair deficient, this class of drugs …
Permanent link to this article: https://inheritedcancer.net/5nls2017/
ICARE Newsletter Winter 2017
Newly Approved PARP-Inhibitor (Rucaparib) to Treat BRCA Carriers with Ovarian Cancer
ICARE Newsletter Winter 2017
Newly Approved PARP-Inhibitor (Rucaparib) to Treat BRCA Carriers with Ovarian Cancer
The FDA just approved another PARP inhibitor, rucaparib, for BRCA carriers with ovarian cancer who have already been treated with two or more chemotherapies. Among those with BRCA-mutant ovarian cancers, 54% had a partial or complete response to the drug with a median duration response of 9.2 months. The agency also approved a companion diagnostic …
Permanent link to this article: https://inheritedcancer.net/6nlw2017/
ICARE Newsletter Winter 2017
NCCN Guidelines Version 1.2017: Genetic/Familial High-Risk Assessment: Breast and Ovarian
ICARE Newsletter Winter 2017
NCCN Guidelines Version 1.2017: Genetic/Familial High-Risk Assessment: Breast and Ovarian
Additional guidance pertaining to cancer risk management was provided in the most recent version of the NCCN Guidelines for inherited breast and ovarian cancer. These guidelines now include an expanded table outlining cancer risks and management for each gene, taking into account the age at initiation of each risk management modality as well as footnotes …
Permanent link to this article: https://inheritedcancer.net/1nlw2017/
ICARE Newsletter Summer 2016
Practice Guideline Updates for NCCN Genetic/Familial High-Risk Assessment
ICARE Newsletter Summer 2016
Practice Guideline Updates for NCCN Genetic/Familial High-Risk Assessment
The National Comprehensive Cancer Network (NCCN) is a network of oncology healthcare providers who work together to develop best practice guidelines for the delivery of cancer care. Given the increasing use of testing for mutations in several inherited cancer genes at one time (called “multi-gene panel testing”), the Breast/Ovarian and Colorectal Panels sought to provide …
Permanent link to this article: https://inheritedcancer.net/1nls2016/
ICARE Newsletter Summer 2016
Practice Guideline Updates for NCCN Genetic/Familial High-Risk Assessment
ICARE Newsletter Summer 2016
Practice Guideline Updates for NCCN Genetic/Familial High-Risk Assessment
The National Comprehensive Cancer Network (NCCN) is a network of oncology healthcare providers who work together to develop best practice guidelines for the delivery of cancer care. Given the increasing use of testing for mutations in several inherited cancer genes at one time (called “multi-gene panel testing”), the Breast/Ovarian and Colorectal Panels sought to provide …
Permanent link to this article: https://inheritedcancer.net/2nls2016/
Permanent link to this article: https://inheritedcancer.net/1nls2015/
ICARE Newsletter Winter 2015
Highlights of the 2014 National Comprehensive Cancer Network (NCCN) Update
ICARE Newsletter Winter 2015
Highlights of the 2014 National Comprehensive Cancer Network (NCCN) Update
Genetic/Familial High-Risk Assessment: Breast and Ovarian Guidelines For breast cancer screening in BRCA carriers, yearly MRI is recommended starting at age 25; mammograms may be considered in instances where MRI is unavailable or individualized based on earliest age of onset in the family. From age 30-75, annual mammogram and breast MRI is recommended. Above age …
Permanent link to this article: https://inheritedcancer.net/5nlw2015/
ICARE Newsletter Winter 2015
Highlights of the 2014 National Comprehensive Cancer Network (NCCN) Update
ICARE Newsletter Winter 2015
Highlights of the 2014 National Comprehensive Cancer Network (NCCN) Update
Genetic/Familial High-Risk Assessment: Colorectal Guidelines Recommendation that tumors from newly diagnosed colorectal cancer patients be screened for Lynch syndrome (called “Universal Tumor Screening”). A new algorithm was created for Routine Tumor Testing Criteria for Lynch Syndrome Surveillance/Management recommendations were refined by gene for the various Lynch Syndrome genes. Management recommendations were refined for other inherited …
Permanent link to this article: https://inheritedcancer.net/6nlw2015/
ICARE Newsletter Winter 2015
The First PARP-Inhibitor to Be Approved for Clinical Use in BRCA Carriers
ICARE Newsletter Winter 2015
The First PARP-Inhibitor to Be Approved for Clinical Use in BRCA Carriers
More frequently, cancer drugs are being developed to treat tumors based on their molecular make-up. PARP inhibitors are the first class of drugs specifically developed to treat BRCA-related tumors through targeting the DNA repair pathway. The PARP Inhibitors target this pathway and cause cancer cells to die while healthy cells are spared. Although PARP inhibitors …
Permanent link to this article: https://inheritedcancer.net/2nlw2015/
ICARE Newsletter Summer 2013
US Preventive Services Task Force (USPSTF) Guidelines for Inherited Breast and Ovarian Cancer and Implications to the Affordable Care Act (ACA)
ICARE Newsletter Summer 2013
US Preventive Services Task Force (USPSTF) Guidelines for Inherited Breast and Ovarian Cancer and Implications to the Affordable Care Act (ACA)
Recently, the USPSTF released updated draft guidelines in April 2013 (from those previously published in 2005) for inherited breast and ovarian cancer due to germline BRCA1 and BRCA2 gene mutations.1 USPSTF is comprised of primary care providers who review the available literature and issue guidelines about risk assessment, testing and management based on available evidence. …
Permanent link to this article: https://inheritedcancer.net/4nls2013/
ICARE Newsletter Summer 2013
BRCA Testing: Supreme Court Update
ICARE Newsletter Summer 2013
BRCA Testing: Supreme Court Update
In a landmark decision regarding the patenting of human genes on Thursday June 13, 2013, the Supreme Court of the United States unanimously ruled that human genes may not be patented. The case specifically concerned the BRCA1 and BRCA2 gene patents, held by the Utah-based company, Myriad Genetics. In the ruling, Justice Clarence Thomas wrote for the …
Permanent link to this article: https://inheritedcancer.net/1nls2013/
ICARE Newsletter Summer 2012
Prostate Cancer Screening Recommendations for Men with BRCA Mutations
ICARE Newsletter Summer 2012
Prostate Cancer Screening Recommendations for Men with BRCA Mutations
Over the last few years, there have been several studies that suggest that men with BRCA mutations are at a higher risk for developing and dying from aggressive prostate cancer. It is possible that PSA testing may be of benefit in men with BRCA mutations. However, until the utility of PSA is determined in these …
Permanent link to this article: https://inheritedcancer.net/2nls2012/