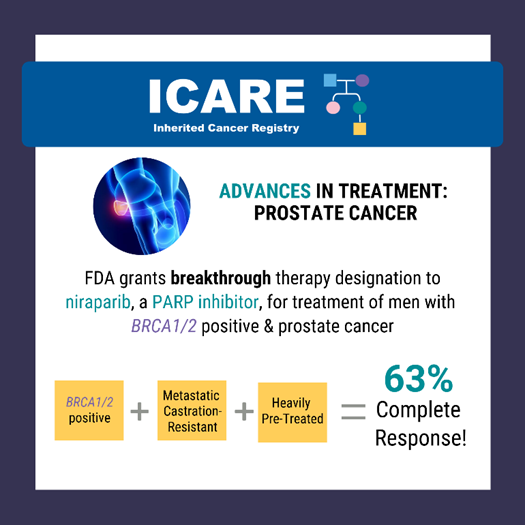
 A recent study reported a high complete response rate among men with a BRCA1/2 mutation with metastatic, castration-resistant prostate cancer who were treated with niraparib (a PARP inhibitor) of 63% compared to 17% in the non-BRCA1/2 group.1 Based on this data, the Federal Drug Administration (FDA) granted breakthrough therapy designation to niraparib on October 3, 2019 to expand the treatment options for men with BRCA1/2 positive, metastatic, castration-resistant prostate cancer.2
A recent study reported a high complete response rate among men with a BRCA1/2 mutation with metastatic, castration-resistant prostate cancer who were treated with niraparib (a PARP inhibitor) of 63% compared to 17% in the non-BRCA1/2 group.1 Based on this data, the Federal Drug Administration (FDA) granted breakthrough therapy designation to niraparib on October 3, 2019 to expand the treatment options for men with BRCA1/2 positive, metastatic, castration-resistant prostate cancer.2
1Smith, et al. Presented at 2019 ESMO Congress. 2019 Sept-Oct. Barcelona, Spain. Abstract LBA50. 2Augenstein S. FDA Grants Breakthrough Therapy Designation to Niraparib for mCRPC. 2019 Oct. Available at: https://tinyurl.com/BRCAassociatedprostatecancer.
Social media post: https://tinyurl.com/ICARE20191220
