
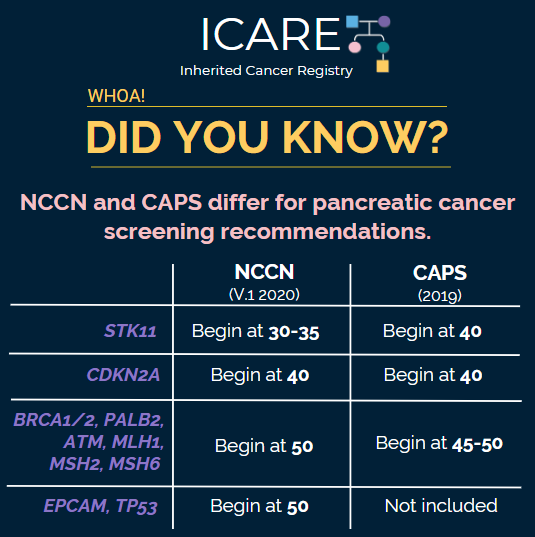
The International Cancer of the Pancreas Screening (CAPS) Consortium recently published updated recommendations about pancreatic cancer screening through MRI/magnetic retrograde cholangiopancreatography (MRCP) and/or an endoscopic ultrasound (EUS).1 Specifically, these guidelines now recommend that individuals with a CDKN2A or STK11 mutation begin screening at age 40. Screening for individuals with a BRCA1/2, ATM, PALB2, MLH1, or MSH2 mutation is only recommended if they have at least one first-degree relative with pancreatic cancer, beginning at age 45-50 or 10 years younger than the youngest relative diagnosed with pancreatic cancer. These guidelines were developed through expert consensus based on existing research; however, there remains a need for more information to understand the benefits and risks of pancreatic cancer screening. Both patients and their treating providers should be aware that these guidelines have some differences from the recently published NCCN genetic/familial breast, ovarian, and pancreatic guidelines, as outlined in the table below.2
| Age to Begin Pancreatic Cancer Screening per NCCN & CAPS | ||
| Gene | NCCN (V.1.2020) | CAPS (2019) |
| STK11 | Begin at 30-35 | Begin at 40 |
| CDKN2A | Begin at 40 | Begin at 40 |
| BRCA1/2, PALB2, ATM, MLH1, MSH2, MSH6 | Begin at 50 | Begin at 45-50 |
| EPCAM, TP53 | Begin at 50 | Not included |
1Goggins, et al. Gut. 2020 Jan. PMID: 31672839; 2NCCN Practice Guidelines. V.1.2020. 2019 Dec. Available at: NCCN.org
Social media post: https://tinyurl.com/ICARE202026
