Big news for the NF1 community: Selumetinib is now FDA-approved for inoperable plexiform neurofibromas. A major step forward in treatment options for Neurofibromatosis Type 1! 🔗 Read the full FDA announcement at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-selumetinib-pediatric-patients-1-year-age-and-older-neurofibromatosis-type-1
Tag: Neurofibromatosis Type 1
Permanent link to this article: https://inheritedcancer.net/post09302025/
Permanent link to this article: https://inheritedcancer.net/post51821/
ICARE Newsletter Summer 2020
Treatment Advances Among Those with Neurofibromatosis Type 1
ICARE Newsletter Summer 2020
Treatment Advances Among Those with Neurofibromatosis Type 1
There continue to be ongoing advances in treatment studies among those with inherited cancer gene mutations, which are rapidly being followed by FDA approval for specific cancer treatments. Select studies and advances are summarized below: Neurofibromatosis Type 1 (NF1): The FDA granted selumetinib (a MEK inhibitor) breakthrough therapy designation for treatment of inoperable plexiform neurofibromas.
Permanent link to this article: https://inheritedcancer.net/8nls2020/
ICARE Social Media Post February 2020
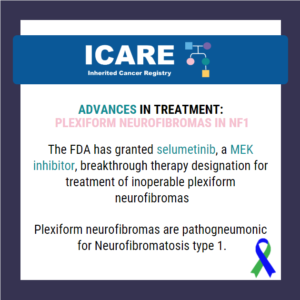
Advances in Treatment: Plexiform Neurofibromas in NF1
ICARE Social Media Post February 2020
Advances in Treatment: Plexiform Neurofibromas in NF1
The FDA has granted a breakthrough therapy designation to selumetinib, a MEK inhibitor, for treatment of inoperable plexiform neurofibromas. These types of neurofibromas are almost exclusively seen in individuals with neurofibromatosis type 1 (NF1). These plexiform neurofibromas are benign tumors on the nerve sheaths and can develop anywhere in the body. These tumors typically cause …
Permanent link to this article: https://inheritedcancer.net/post22820/