Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic Cancer – Released February 12th, 2024 (V3.2024)
Check out the full guidelines by creating a FREE account at www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf
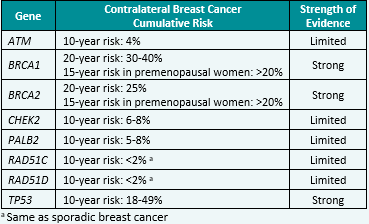
Contralateral breast cancer risks in these updated guidelines:
Expanded guidance about gynecologic cancers in BRCA1/2 carriers:
- Reproductive considerations
- Non-surgical and surgical risk reduction
- Salpingectomy
- Hysterectomy considerations
- Hormone replacement therapy (HRT) after risk-reducing removal of the ovaries
Some highlights related to HRT include:
- Premature menopause due to risk-reducing removal of the ovaries and fallopian tubes can cause detriments to quality-of-life, as well as bone, cardiovascular, psychosocial, neurologic, and sexual health.
- HRT can reduce these risks and is generally not contraindicated.
- Thus, HRT should be discussed with premenopausal patients who do not have a personal history of breast cancer.
Genetic/Familial High-Risk Assessment: Colorectal Cancer – Released October 30th, 2023 (V2.2023)
Check out the full guidelines by creating a FREE account at www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf
- Test Selection modified: Germline panel testing should include at a minimum the following colorectal cancer risk-associated genes: APC, MUTYH, MLH1, MSH2, MSH6, PMS2, EPCAM, BMPR1A, SMAD4, PTEN, STK11, TP53
- MUTYH monoallelic pathogenic variant/heterozygotes (carrier): revised to indicate “No increased risk.”
- Criteria for the Evaluation of Lynch Syndrome Based on Personal or Family History of Cancer were expanded from personal history of colorectal and/or endometrial cancer to any Lynch Syndrome-related cancer.
- Please see guidelines for many additional revisions based on tumor screening results; and refinement in care based on having an inherited colorectal cancer predisposing gene.
