This question was addressed by Ronald D. Alvarez, MD, MBA, Professor and Chairman of the Department of Obstetrics and Gynecology at the Vanderbilt University Medical Center in Nashville, Tennessee. He is also the current vice chair of the National Comprehensive Cancer Network (NCCN) Ovarian Cancer Treatment Guidelines and has served in multiple leadership roles in …
Tag: Hormonal Replacement Therapy
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2024-ask-the-expert/
ICARE Newsletter Spring 2024
National Comprehensive Cancer Network (NCCN) Guideline Updates
ICARE Newsletter Spring 2024
National Comprehensive Cancer Network (NCCN) Guideline Updates
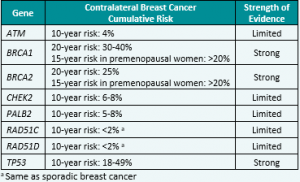
Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic Cancer – Released February 12th, 2024 (V3.2024) Check out the full guidelines by creating a FREE account at www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf Contralateral breast cancer risks in these updated guidelines: Expanded guidance about gynecologic cancers in BRCA1/2 carriers: Some highlights related to HRT include: Genetic/Familial High-Risk Assessment: Colorectal Cancer – Released …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2024-national-comprehensive-cancer-network-nccn-guideline-updates/
ICARE Newsletter Fall 2022
BRCA1/2 Cancer Risk Updates
ICARE Newsletter Fall 2022
BRCA1/2 Cancer Risk Updates
During preventive surgery to remove the ovaries and fallopian tubes (called a risk-reducing salpingo-oophorectomy orRRSO), a new study found that the detection of tubal intraepithelial carcinoma predicts the risk of later peritonealcancer.1 These findings show the importance of timely RRSO and the need to do a careful pathology exam of the ovaries and fallopian tubes …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2022-brca1-2-cancer-risk-updates/
ICARE Social Media Post September 2022
Hormonal Replacement Therapy (HRT) in BRCA1 and BRCA2 carriers
ICARE Social Media Post September 2022
Hormonal Replacement Therapy (HRT) in BRCA1 and BRCA2 carriers
A recent study showed hormone replacement therapy (HRT) is reasonable to offer to BRCA1 and BRCA2 carriers who underwent risk-reducing salpingo-oophorectomy (RSSO). Read the full article at this link: https://pubmed.ncbi.nlm.nih.gov/32143914/Reference: Mills, et al. Gynecol Oncol; 2020 Jun;157(3):706-710. PMID: 32143914.
Permanent link to this article: https://inheritedcancer.net/post2922/