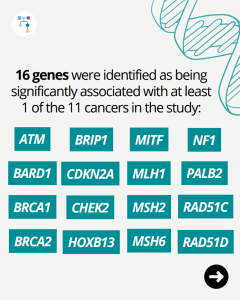
Learn more by reading the full article at: https://jamanetwork.com/journals/jamaoncology/article-abstract/2838068#xd_co_f=MTdiMGE0YjctNzA5ZC00YTFiLTkzMmQtNzBkZDIzZDg0NzEz~ Reference: Shevach et al. JAMA Oncol. 2025:e252879. PMID: 40875208.
Tag: Multi-Gene Panels
Permanent link to this article: https://inheritedcancer.net/post10072025/
ICARE Social Media Post June 2025 2025
PREMM5: Model to Estimate the Risk for Having Lynch Syndrome
ICARE Social Media Post June 2025 2025
PREMM5: Model to Estimate the Risk for Having Lynch Syndrome
PREMM5 is a model to estimate the risk for having Lynch Syndrome. PREMMplus is a model that estimates risks in 19-cancer risk genes, including Lynch Syndrome genes, BRCA, and other genes. A new study that compared PREMM5 and PREMMplus found that PREMMplus was just as good as PREMM5 in identifying patients with Lynch Syndrome. PREMMplus …
Permanent link to this article: https://inheritedcancer.net/post060325/
ICARE Newsletter Fall 2024
American Society of Clinical Oncology (ASCO) Guideline Update: Selecting Genetic Tests in Patients with Various Cancers
ICARE Newsletter Fall 2024
American Society of Clinical Oncology (ASCO) Guideline Update: Selecting Genetic Tests in Patients with Various Cancers
What elements are most important in the collection of family history? When and how to use multigene panel testing when indicated? Which genes should be tested based on cancer type? Among patients with tumor testing, who should be offered germline testing? For a full list of recommendations in this guideline, the article is available at: …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2024-american-society-of-clinical-oncology-asco-guideline-update-selecting-genetic-tests-in-patients-with-various-cancers/
Permanent link to this article: https://inheritedcancer.net/post81621/
Permanent link to this article: https://inheritedcancer.net/post72721/
Permanent link to this article: https://inheritedcancer.net/post61521/
ICARE Social Media Post September 2020
NCCN Genetic Testing Choice Updates
ICARE Social Media Post September 2020
NCCN Genetic Testing Choice Updates
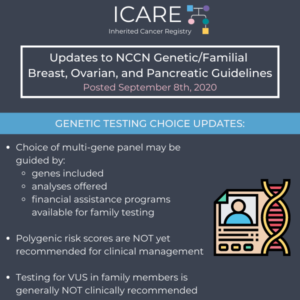
The National Comprehensive Cancer Network (NCCN) released new guidelines on September 8th, 2020, which included updates to genetic testing choices and considerations as follows: Choice of multi-gene panel may be guided by genes included, analyses offered, and financial assistance programs available for family testing Significant limitations in interpretations of polygenic risk scores: NOT recommended for …
Permanent link to this article: https://inheritedcancer.net/post91120/
ICARE Social Media Post January 2020
Celebrating 10 Years of ICARE
ICARE Social Media Post January 2020
Celebrating 10 Years of ICARE
Happy New Year! 2020 represents a decade for ICARE We are celebrating 10 years of research, education, and engagement, through which we have enrolled nearly 3500 participants, including over 2000 gene mutation carriers, disseminated 15 newsletters, led and collaborated on multiple research projects, and impacted individuals affected by inherited cancer predisposition all over the …
Permanent link to this article: https://inheritedcancer.net/post1920/
ICARE Newsletter Summer 2019
Expanding Our Thinking About Cancer Risks in TP53 Mutations and Li-Fraumeni Syndrome
ICARE Newsletter Summer 2019
Expanding Our Thinking About Cancer Risks in TP53 Mutations and Li-Fraumeni Syndrome
Since expanded genetic testing has become available through multigene panel tests, studies have suggested that many people identified to have TP53 mutations do not have a typical personal or family history, which is usually seen with Li-Fraumeni syndrome (LFS). A recent study looking at over 300 individuals with TP53 mutations (identified through multi-gene panel testing) …
Permanent link to this article: https://inheritedcancer.net/11nls2019/
ICARE Newsletter Winter 2018
Advances in Cancer Screening Among Li-Fraumeni Syndrome Patients
ICARE Newsletter Winter 2018
Advances in Cancer Screening Among Li-Fraumeni Syndrome Patients
Several research groups from around the world that have conducted cancer screening among patients with Li-Fraumeni syndrome and a germline TP53 mutation have recently reported on their observations. Specifically, the National Cancer Institute group demonstrated that screening inclusive of rapid total body MRI detected cancers at an early stage,1 similar to findings published through other …
Permanent link to this article: https://inheritedcancer.net/5nlw2018/
Permanent link to this article: https://inheritedcancer.net/2nls2017/
ICARE Newsletter Summer 2012
Emerging Cancer Panels for Testing Patients for Inherited Cancer Predisposition
ICARE Newsletter Summer 2012
Emerging Cancer Panels for Testing Patients for Inherited Cancer Predisposition
Genetic testing for inherited cancer predisposition is typically performed by testing for one condition at a time. However, with the tremendous advances in genetic testing technologies over the last few years, the cost of testing has plummeted. To put this into perspective, the first human genome cost 2-3 billion dollars to sequence and took over …
Permanent link to this article: https://inheritedcancer.net/1nls2012/