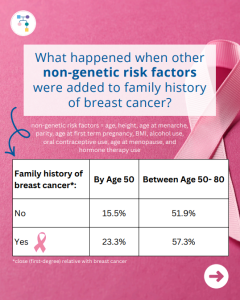
A new study reports that risks for breast cancer in BRCA1 carriers did NOT markedly differ based on family history. However, additional non-genetic risk factors were important modifiers of risk. To learn more read the article at: https://jamanetwork.com/journals/jamaoncology/article-abstract/2839917 Reference: O’Brien, et al. JAMA Oncol. 2025. Online ahead of print. PMID: 41066089.
Tag: Family Impact
Permanent link to this article: https://inheritedcancer.net/post11242025/
ICARE Social Media Post November 2025
ATM: How much does breast cancer family history and non-genetic risk factors affect risk?
ICARE Social Media Post November 2025
ATM: How much does breast cancer family history and non-genetic risk factors affect risk?
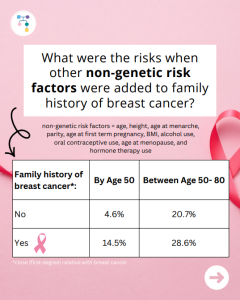
A new study evaluated breast cancer risks in ATM carriers based on family history and found that risks for breast cancer were higher in those with a family history and additional non-genetic risk factors modified risks in those who already had a family history. To learn more read the article at: https://jamanetwork.com/journals/jamaoncology/article-abstract/2839917 Reference: O’Brien, et …
Permanent link to this article: https://inheritedcancer.net/post11212025/
ICARE Social Media Post November 2025
CHEK2: How much does breast cancer family history and non-genetic risk factors affect risk?
ICARE Social Media Post November 2025
CHEK2: How much does breast cancer family history and non-genetic risk factors affect risk?
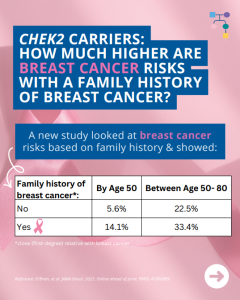
A new study looked at breast cancer risks in CHEK2 carriers based on family history and found that risks for breast cancer were much higher in those with a family history and additional non-genetic risk factors further modified risks. To learn more read the article at: https://jamanetwork.com/journals/jamaoncology/article-abstract/2839917 Reference: O’Brien, et al. JAMA Oncol. 2025. Online …
Permanent link to this article: https://inheritedcancer.net/post11192025/
Permanent link to this article: https://inheritedcancer.net/post11172025/
ICARE Social Media Post November 2025
BRCA2: How much does breast cancer family history and non-genetic risk factors affect risk?
ICARE Social Media Post November 2025
BRCA2: How much does breast cancer family history and non-genetic risk factors affect risk?
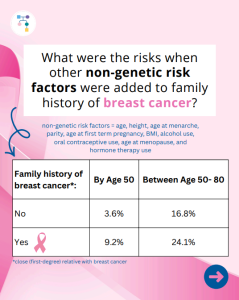
A new study reports that risks for breast cancer in BRCA2 carriers were higher in those with a family history, while additional non-genetic risk factors seemed to minimally modify risks. To learn more read the article at: https://jamanetwork.com/journals/jamaoncology/article-abstract/2839917 Reference: O’Brien, et al. JAMA Oncol. 2025. Online ahead of print. PMID: 41066089.
Permanent link to this article: https://inheritedcancer.net/post11142025/
ICARE Newsletter Fall 2025
ICARE Community Spotlight: Kathy Baker
ICARE Newsletter Fall 2025
ICARE Community Spotlight: Kathy Baker
I was only 30 when my 32-year-old sister was diagnosed with breast cancer. A couple years later, when a mobile mammography bus showed up at my law school offering free mammograms, I decided it couldn’t hurt to be screened. When my mammogram was normal, I made plans to wait until age 50 for my next …
Permanent link to this article: https://inheritedcancer.net/nlf202512/
ICARE Newsletter Spring 2025
BRCA1/2: Pancreatic Cancer Risks in Women
ICARE Newsletter Spring 2025
BRCA1/2: Pancreatic Cancer Risks in Women
A new international study in over 8000 BRCA1/2 carriers, which included data from ICARE participants, showed risk of pancreatic cancer to age 80 in BRCA1 was 2.2% (95% CI: 1.1%-4.3%) and in BRCA2 was 2.7% (95% CI: 1.3%-5.2%). Of the 34 BRCA1/2 carriers with pancreatic cancer, only 2 had a close (first-degree) relative with pancreatic …
Permanent link to this article: https://inheritedcancer.net/9nls2025/
ICARE Featured Video April 2025
Increasing Cascade Testing: ConnectMyVariant
ICARE Featured Video April 2025
Increasing Cascade Testing: ConnectMyVariant
Below is a featured video from the April 2025 case conference, during which Brian Shirts, MD, PhD from Vanderbilt University Medical Center and Rachel Pearlman, MS, CGC from The Ohio State University highlight how connecting with relatives through publicly available platforms like ConnectMyVariant can lead to increased cascade testing
Permanent link to this article: https://inheritedcancer.net/video41025/
ICARE Featured Video December 2024
Coping with Hereditary Cancer
ICARE Featured Video December 2024
Coping with Hereditary Cancer
Below is a featured video from the December 2024 case conference, during which Lora Thompson, PhD from Moffitt Cancer Center discusses coping with hereditary cancer. Dr. Thompson was joined in commentary by BRCA carriers, Marleah Dean Kruzel, PhD (University of South Florida) and Kathy Baker (My Faulty Gene), as well as panelists Deborah Cragun, PhD, MS, CGC …
Permanent link to this article: https://inheritedcancer.net/video121224/
ICARE Social Media Post October 2024
NCCN BOP Guideline Update #2: Testing Unaffected Family Members
ICARE Social Media Post October 2024
NCCN BOP Guideline Update #2: Testing Unaffected Family Members
The National Comprehensive Cancer Network (NCCN) released updated Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic Cancer guidelines on September 11th, 2024. In these updates, NCCN clarified that it is appropriate to test unaffected (not best testable) family members when they meet testing criteria. To read more, you can check out the full guidelines by creating …
Permanent link to this article: https://inheritedcancer.net/100424_1/
ICARE Newsletter Fall 2024
Community Spotlight: The Patient and the Researcher Shares Her Uncertain Future and Lessons She’s Learned
ICARE Newsletter Fall 2024
Community Spotlight: The Patient and the Researcher Shares Her Uncertain Future and Lessons She’s Learned
By Marleah Dean Kruzel, PhD, BRCA2 Previvor When I was eight years old, my mother found a lump in her breast – barely noticeable. For a few years, I watched her undergo chemotherapy, radiation, and a prophylactic mastectomy and reconstruction. Since then, my maternal aunt and grandmother also fought breast cancer, and we learned my …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2024-community-spotlight-the-patient-and-the-researcher-shares-her-uncertain-future-and-lessons-shes-learned/
ICARE Featured Video July 2024
Coping with Hereditary Cancer: Managing Emotional Distress and Communicating with Family
ICARE Featured Video July 2024
Coping with Hereditary Cancer: Managing Emotional Distress and Communicating with Family
Below is a featured video from the July 2024 patient forum, during which patients had the opportunity to ask questions and discuss how to manage emotional distress and communicate with family about inherited cancer predisposition with our expert panelists.
Permanent link to this article: https://inheritedcancer.net/video70924/
ICARE Newsletter Fall 2023
Community Spotlight
ICARE Newsletter Fall 2023
Community Spotlight
My paternal grandparents were my heroes. Wise beyond their time, they relished teaching our familythat knowledge is power, health is everything, and love is unconditional. Back then, Prevention healthmagazine and vitamin supplements filled their mailbox and 1960’s exercise guru Jack LaLane, and health foodadvocate Euell Gibbons, beckoned new followers from a talking picture box in …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2023-community-spotlight/
ICARE Newsletter Fall 2023
Germline EGFR Mutations and Familial Lung Cancer
ICARE Newsletter Fall 2023
Germline EGFR Mutations and Familial Lung Cancer
A study, in which our Vanderbilt colleagues Georgia Wiesner, MD, MS (geneticist) and Kelly Taylor, MS, LCGC (genetic counselor) participated, was recently published about the inherited T790M EGFR mutation. Mutations in this gene lead to a higher risk or lung cancer and were found to be more common in the Southeast United States where there …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2023-germline-egfr-mutations-and-familial-lung-cancer/
ICARE Newsletter Fall 2023
Newly released ACMG Clinical Practice Resource on CHEK2 Developed Through a Group of Worldwide Experts!
ICARE Newsletter Fall 2023
Newly released ACMG Clinical Practice Resource on CHEK2 Developed Through a Group of Worldwide Experts!
A person with a pathogenic variant in the CHEK2 gene may be at an increased risk for developing breast and other cancers. This ACMG Clinical Practice Resource, published in ACMG’s flagship journal, Genetics in Medicine, provides valuable information for healthcare providers caring for individuals with pathogenic variants in the CHEK2gene. This new ACMG Practice Resource …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2023-newly-released-acmg-clinical-practice-resource-on-chek2-developed-through-a-group-of-worldwide-experts/
Other Cancer Genetics Webinar April 2023
Communicating about Inherited Cancer Risk, Uncertainty, and Decision Making
Other Cancer Genetics Webinar April 2023
Communicating about Inherited Cancer Risk, Uncertainty, and Decision Making
Below you may watch an exciting presentation about communicating about inherited cancer risk, uncertainty, and decision making by Marleah Dean Kruzel, PhD from the University of South Florida.
Permanent link to this article: https://inheritedcancer.net/video40423/
ICARE Social Media Post September 2021
USA Today Article: Fighting Cancer with Your Own Family History
ICARE Social Media Post September 2021
USA Today Article: Fighting Cancer with Your Own Family History
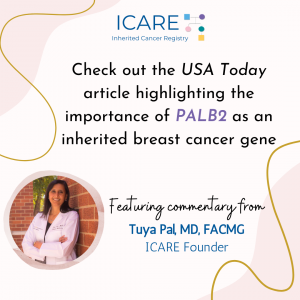
Check out the full 𝘜𝘚𝘈 𝘛𝘰𝘥𝘢𝘺 article, featuring commentary from Dr. Tuya Pal (ICARE Founder), highlighting the importance of PALB2 as an inherited breast cancer gene: https://www.futureofpersonalhealth.com/breast-health/fighting-cancer-with-your-own-family-history/# Additional guidance is available through an impactful PALB2 practice resource recently published through ACMG: https://www.acmg.net/PDFLibrary/41436_2021_1151_OnlinePDF.pdf Reference: Tischkowitz, et al. Genet Med. 2021 Aug;23(8):1416-1423. PMID: 33976419
Permanent link to this article: https://inheritedcancer.net/post92221/
ICARE Social Media Post May 2021
CDKN2A: Cancer Risks and Risk Management
ICARE Social Media Post May 2021
CDKN2A: Cancer Risks and Risk Management
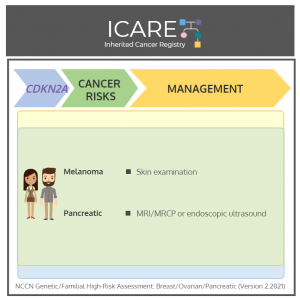
𝘐𝘯 𝘳𝘦𝘤𝘰𝘨𝘯𝘪𝘵𝘪𝘰𝘯 𝘰𝘧 𝘴𝘬𝘪𝘯 𝘤𝘢𝘯𝘤𝘦𝘳 𝘢𝘸𝘢𝘳𝘦𝘯𝘦𝘴𝘴 𝘮𝘰𝘯𝘵𝘩, we present cancer risks and management for 𝗖𝗗𝗞𝗡𝟮𝗔 per National Comprehensive Cancer Network (NCCN) Genetic/Familial High-Risk Assessment: Version 2.2021 𝗠𝗲𝗻 & 𝗪𝗼𝗺𝗲𝗻:Melanoma risk: Elevated at 28-67% – Recommend annual full-body skin exam, regular self-examinations, and routine sun protective behaviors. Pancreatic cancer risk: >15% – Consider MRI/MRCP and/or endoscopic …
Permanent link to this article: https://inheritedcancer.net/post51321/
Permanent link to this article: https://inheritedcancer.net/post40221/
ICARE Social Media Post March 2021
STK11: Cancer Risks and Risk Management
ICARE Social Media Post March 2021
STK11: Cancer Risks and Risk Management
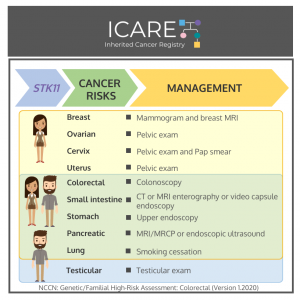
Gene: STK11 Cancer Risks and Management per National Comprehensive Cancer Network (NCCN) Genetic/Familial High-Risk Assessment: Colorectal Version 1.2020 Women:Breast cancer risk: Elevated at 40-60% – Recommend annual mammogram and breast MRI starting at around age 30. Ovarian tumor risk (typically benign sex cord/Sertoli cell tumors): Elevated at 18-21% – Recommend annual pelvic exam starting at …
Permanent link to this article: https://inheritedcancer.net/post33021/
ICARE Social Media Post March 2021
MUTYH: Cancer Risks and Risk Management
ICARE Social Media Post March 2021
MUTYH: Cancer Risks and Risk Management
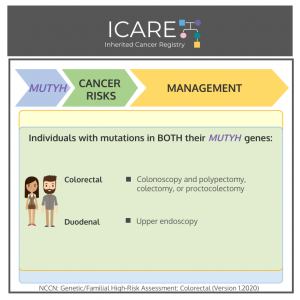
Gene: MUTYH Cancer Risks and Management per National Comprehensive Cancer Network (NCCN) Genetic/Familial High-Risk Assessment: Colorectal Version 1.2020 Men & women with two mutations in MUTYH:Colon cancer risk: High risk – Recommend colonoscopy every 1-2 years beginning at age 25-30; colectomy considered based on polyp burden and age. Duodenal cancer risk: Elevated – Consider baseline …
Permanent link to this article: https://inheritedcancer.net/post32321/
ICARE Social Media Post March 2021
APC: Cancer Risks and Risk Management
ICARE Social Media Post March 2021
APC: Cancer Risks and Risk Management
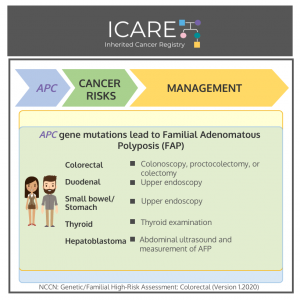
𝗚𝗲𝗻𝗲: 𝗔𝗣𝗖 Cancer Risks and Management per National Comprehensive Cancer Network (NCCN) Genetic/Familial High-Risk Assessment: Colorectal Version 1.2020 𝗠𝗲𝗻 & 𝗪𝗼𝗺𝗲𝗻:𝘈𝘗𝘊 mutation leading to classic form of Familial Adenomatous Polyposis (FAP):Colorectal cancer risk: >99% if untreated – Treatment is based on polyp burden and includes proctocolectomy (with subsequent endoscopic screening of the ileal pouch) or …
Permanent link to this article: https://inheritedcancer.net/post31621/
ICARE Newsletter Winter 2021
Learning You Have a Mutation in an Inherited Cancer Gene: What’s Next?
ICARE Newsletter Winter 2021
Learning You Have a Mutation in an Inherited Cancer Gene: What’s Next?
The benefits achieved through genetic testing for inherited cancer only happen by acting upon the results. This can be through guiding cancer treatment, receiving appropriate cancer risk management strategies, and sharing results with at-risk family members so they too can benefit from this information. We recently reported on results of our study, made possible through …
Permanent link to this article: https://inheritedcancer.net/6nlw2021/
ICARE Social Media Post January 2021
Family Sharing Resources: GeneSHARE
ICARE Social Media Post January 2021
Family Sharing Resources: GeneSHARE
With the tremendous advances in gene-based care among those at risk for inherited cancer, we are trying to develop and improve tools and strategies to make it easier for more people to benefit from genetic testing. We are excited to share with you a free online toolkit called 𝗚𝗲𝗻𝗲𝗦𝗛𝗔𝗥𝗘, which is aimed at helping patients …
Permanent link to this article: https://inheritedcancer.net/post10821/
ICARE Publication January 2021
Sharing genetic test results with family members of BRCA, PALB2, CHEK2, and ATM carriers
ICARE Publication January 2021
Sharing genetic test results with family members of BRCA, PALB2, CHEK2, and ATM carriers
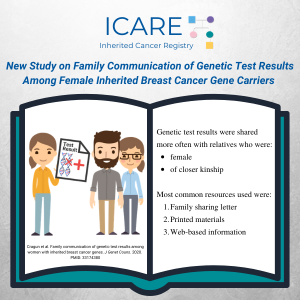
Abstract Objective: This study explored motivators and challenges/barriers to sharing personal genetic test results (GTR) with family members (FM). Methods: Semi-structured, in-depth interviews were conducted with 62 women who had a pathogenic or likely pathogenic (P/LP) variant in a BRCA, PALB2, CHEK2, or ATM gene. Selective qualitative data analysis focused on eliciting motivators and challenges/barriers …
Permanent link to this article: https://inheritedcancer.net/pub10521/
ICARE Social Media Post August 2020
Inherited Prostate Cancer Risk
ICARE Social Media Post August 2020
Inherited Prostate Cancer Risk
Over 600,000 men age 40 and older who were part of a family with at least three consecutive generations affected with prostate cancer were studied from the Utah Population Database. Findings from this study showed that: 36,000 had prostate cancer (5.9%) 2,500 had early-onset disease (7%) 4,000 had lethal disease (11.1%) 15,000 had clinically significant …
Permanent link to this article: https://inheritedcancer.net/post81420/
ICARE Publication July 2020
Update on multi-gene panel testing and communication of genetic test results
ICARE Publication July 2020
Update on multi-gene panel testing and communication of genetic test results
Abstract With technological advances, multi-gene panel testing has become increasingly used to identify patients at risk for hereditary breast cancer (HBC). There are currently evidence-based interventions and breast cancer screening strategies that exist for cancer prevention and early detection among patients with HBC. Moreover, in addition to the personal impact of identifying HBC, this information …
Permanent link to this article: https://inheritedcancer.net/pub70820/
ICARE Social Media Post April 2020
PALB2: Cancer Risks and Risk Management
ICARE Social Media Post April 2020
PALB2: Cancer Risks and Risk Management
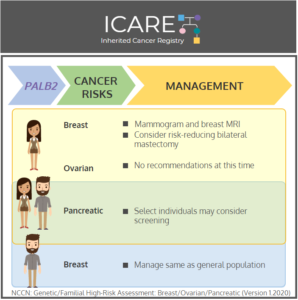
Gene: PALB2 Cancer Risks and Management (per NCCN version 1.2020): Women: Breast cancer risk: Elevated at 53% – Recommend annual breast MRI with contrast starting at age 30, and annual mammogram with consideration of tomosynthesis starting at age 30; Consider risk-reducing mastectomy. Ovarian cancer risk: Elevated at 5% – Manage based on family history. Men …
Permanent link to this article: https://inheritedcancer.net/post42820/
ICARE Social Media Post April 2020
BRCA2: Cancer Risks and Risk Management
ICARE Social Media Post April 2020
BRCA2: Cancer Risks and Risk Management
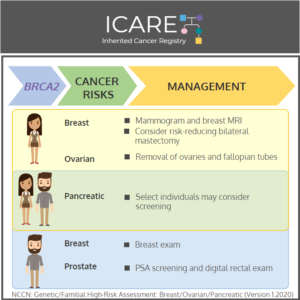
Gene: BRCA2 Cancer Risks and Management (per NCCN version 3.2019): Women: Breast cancer risk: Elevated at 60%-70% – Recommend clinical breast exam every 6-12 months starting at age 25, annual breast MRI with contrast starting at age 25, and annual mammogram with consideration of tomosynthesis starting at age 30; consider risk-reducing mastectomy. Ovarian cancer risk: …
Permanent link to this article: https://inheritedcancer.net/post42120/
ICARE Social Media Post April 2020
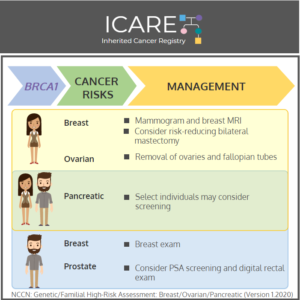
BRCA1: Cancer Risks and Risk Management
ICARE Social Media Post April 2020
BRCA1: Cancer Risks and Risk Management
Gene: BRCA1 Cancer Risks and Management (per NCCN version 3.2019): Women: Breast cancer risk: Elevated at 60%-70% – Recommend clinical breast exam every 6-12 months starting at age 25, annual breast MRI with contrast starting at age 25, and annual mammogram with consideration of tomosynthesis starting at age 30; consider risk-reducing mastectomy. Ovarian cancer risk: …
Permanent link to this article: https://inheritedcancer.net/post41420/
ICARE Social Media Post April 2020
EPCAM: Cancer Risks and Risk Management
ICARE Social Media Post April 2020
EPCAM: Cancer Risks and Risk Management
Gene: EPCAM Cancer Risks and Management (per NCCN version 3.2019): Women: Endometrial cancer risk: Elevated at 21%-57% – Consider risk-reducing hysterectomy. Ovarian cancer risk: Elevated at 10%-38% – Recommend risk-reducing bilateral salpingo-oophorectomy (removal of ovaries and fallopian tubes). Men and Women: Colorectal cancer risk: Elevated at 43%-52% – Recommend colonoscopy every 1-2 years starting at …
Permanent link to this article: https://inheritedcancer.net/post4120/
ICARE Social Media Post March 2020
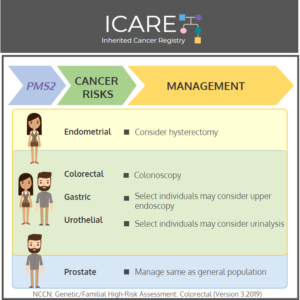
PMS2: Cancer Risks and Risk Management
ICARE Social Media Post March 2020
PMS2: Cancer Risks and Risk Management
Gene: PMS2 Cancer Risks and Management (per NCCN version 3.2019): Women: Endometrial cancer risk: Elevated at 0%-15% – Consider risk-reducing hysterectomy. Men and Women: Colorectal cancer risk: Elevated at 12%-20% – Recommend colonoscopy every 1-2 years starting at age 20-25 Gastric cancer risk: Not well established – Consider upper endoscopy every 3-5 years beginning at …
Permanent link to this article: https://inheritedcancer.net/post32420/
ICARE Social Media Post March 2020
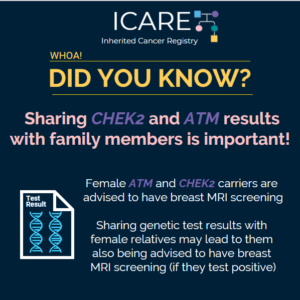
Study Based on ICARE Participants with ATM and CHEK2 Mutations
ICARE Social Media Post March 2020
Study Based on ICARE Participants with ATM and CHEK2 Mutations
Women with ATM and CHEK2 mutations have a lifetime breast cancer risk greater than 20%, which is the threshold at which screening through a breast MRI is recommended. A recently published study based on ICARE participants with ATM and CHEK2 mutations suggested that most female family members of ATM and CHEK2 mutation carriers do not …
Permanent link to this article: https://inheritedcancer.net/post32020/
ICARE Social Media Post March 2020
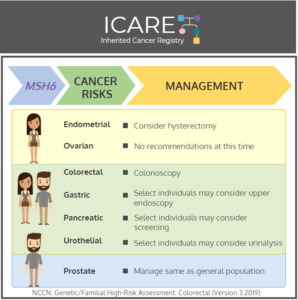
MSH6: Cancer Risks and Risk Management
ICARE Social Media Post March 2020
MSH6: Cancer Risks and Risk Management
Gene: MSH6 Cancer Risks and Management (per NCCN version 3.2019): Women: Endometrial cancer risk: 17%-46% – Consider risk-reducing hysterectomy. Ovarian cancer risk: 1%-11% – Evidence is insufficient to make specific recommendations. Men and Women: Colorectal cancer risk: 15%-44% – Recommend colonoscopy every 1-2 years starting at age 20-25. Gastric cancer risk: 0%-5% – Consider upper …
Permanent link to this article: https://inheritedcancer.net/post31720/
ICARE Social Media Post March 2020
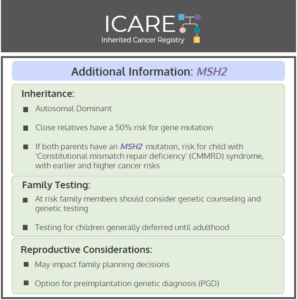
MSH2: Cancer Risks and Risk Management
ICARE Social Media Post March 2020
MSH2: Cancer Risks and Risk Management
Gene: MSH2 Cancer Risks and Management (per NCCN version 3.2019): Women: Endometrial cancer risk: Elevated at 21%-57% – Consider risk-reducing hysterectomy. Ovarian cancer risk: Elevated at 10%-38% – Recommend risk-reducing bilateral salpingo-oophorectomy (removal of ovaries and fallopian tubes). Men and Women: Colorectal cancer risk: Elevated at 43%-52% – Recommend colonoscopy every 1-2 years starting at …
Permanent link to this article: https://inheritedcancer.net/post31020/
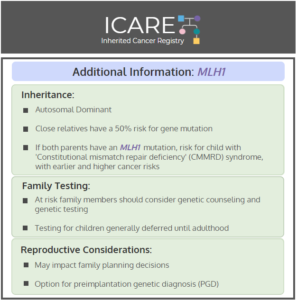
ICARE Social Media Post March 2020
MLH1: Cancer Risks and Risk Management
ICARE Social Media Post March 2020
MLH1: Cancer Risks and Risk Management
Gene: MLH1 Cancer Risks and Management (per NCCN version 3.2019): Women: Endometrial cancer risk: Elevated at 43%-57% – Consider risk-reducing hysterectomy. Ovarian cancer risk: Elevated at 5%-20% – Recommend risk-reducing bilateral salpingo-oophorectomy (removal of ovaries and fallopian tubes). Breast cancer risk: Elevated at 12%-17% – Manage same as general population. Men and Women: Colorectal cancer …
Permanent link to this article: https://inheritedcancer.net/post3320/
ICARE Newsletter Winter 2020
New Study Based on ICARE Participants with ATM & CHEK2 Mutations
ICARE Newsletter Winter 2020
New Study Based on ICARE Participants with ATM & CHEK2 Mutations
We are excited to tell you about our recently published results based solely on data from ICARE participants with ATM and CHEK2 mutations. Our findings suggest most female family members of ATM and CHEK2 mutation carriers do not qualify for breast MRI screening based on family cancer history alone. This emphasizes the need to share …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-winter-2020new-study-based-on-icare-participants-with-atm-chek2-mutations/
ICARE Social Media Post October 2019
Male Breast Cancer Risk
ICARE Social Media Post October 2019
Male Breast Cancer Risk
Did you know? Beyonce’s father, Matthew Knowles, was diagnosed with breast cancer. He states, “we used to think this was only an issue for women, but this is male or female.” According to CBS news, “he is hoping that sharing his story as man with breast cancer will shine a light on the risk men …
Permanent link to this article: https://inheritedcancer.net/post10619/
ICARE Newsletter Winter 2017
How Does Having a Mother with Breast Cancer and a BRCA Mutation Affect Adolescent Girls?
ICARE Newsletter Winter 2017
How Does Having a Mother with Breast Cancer and a BRCA Mutation Affect Adolescent Girls?
A recent study compared psychosocial adjustment and risk perception among 11 to 19 year old daughters of women with breast cancer, comparing those with a BRCA mutation versus those without.1 The overall findings from the study were reassuring, suggesting that adolescent girls from BRCA-positive families had higher self-esteem and similar psychosocial adjustment compared to their …
Permanent link to this article: https://inheritedcancer.net/5nlw2017/
ICARE Newsletter Winter 2016
The Importance of Sharing Genetic Test Results with Family Members
ICARE Newsletter Winter 2016
The Importance of Sharing Genetic Test Results with Family Members
Once an individual has had genetic testing for inherited cancer predisposition this information could help their close family members. For example, when a BRCA mutation or a mutation in another inherited cancer gene is found, it is important for close family members (with or without a diagnosis of cancer) to know so they too can …
Permanent link to this article: https://inheritedcancer.net/1nlw2016/