A new study evaluated breast cancer risks in BARD1 carriers based on family history and found that risks for breast cancer were higher in those with a family history and additional non-genetic risk factors were important modifiers of risk. To learn more read the article at: https://jamanetwork.com/journals/jamaoncology/article-abstract/2839917 Reference: O’Brien, et al. JAMA Oncol. 2025. Online …
Tag: BARD1
Permanent link to this article: https://inheritedcancer.net/post11172025/
ICARE Social Media Post October 2025
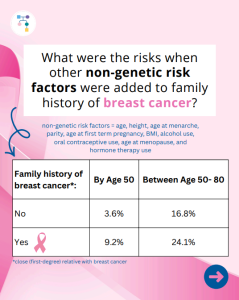
BRCA1/2, PALB2, CHEK2, & ATM: How does family history of breast cancers affect risk?
ICARE Social Media Post October 2025
BRCA1/2, PALB2, CHEK2, & ATM: How does family history of breast cancers affect risk?
In a new study of almost 70,000 women, including those with hereditary forms of breast cancer, breast cancer risk was higher in those with a family history of breast cancer. Family history raised breast cancer risks: ✨Why is this important? ✨This type of information to personalize risk estimates can help women to guide screening and …
Permanent link to this article: https://inheritedcancer.net/post10282025/
Apr 09
ICARE Newsletter Spring 2024
New Guidelines Released Through ASCO-Society of Oncology; Germline Testing in Patients with Breast Center
ICARE Newsletter Spring 2024
New Guidelines Released Through ASCO-Society of Oncology; Germline Testing in Patients with Breast Center
- By admin in Newsletter Articles & Posts
-
April 9, 2024
In January 2024, the American Society of Clinical Oncology (ASCO) in conjunction with the Society of Surgical Oncology released new guidelines for germline testing in patients with breast cancer, which include the following: For a full list of recommendations in this guideline, the article is available at: https://ascopubs.org/doi/10.1200/JCO.23.02225 Bedrosian, et al. J Clin Oncol. 2024;42(5):584-604. PMID:38175972. Social …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2024-new-guidelines-released-through-asco-society-of-oncology-germline-testing-in-patients-with-breast-center/
ICARE Social Media Post January 2023
New ASCO Germline Testing in Patients With Breast Cancer Guidelines
ICARE Social Media Post January 2023
New ASCO Germline Testing in Patients With Breast Cancer Guidelines
The American Society of Clinical Oncology (ASCO) just released new guidelines for germline testing in patients with breast cancer, which include the following:🧬 BRCA1/2 testing should be offered to ALL patients diagnosed with breast cancer at or below age 65🧬 Testing for other hereditary cancer genes should also be offered based on personal and family …
- ATM, BARD1, BRCA1, BRCA2, Breast Cancer, Cancer Risks, CDH1, CHEK2, Genetic Testing, PALB2, PTEN, RAD51C, RAD51D, Social Media, STK11, TP53
Permanent link to this article: https://inheritedcancer.net/post10524/
ICARE Social Media Post October 2023
What Are Established Cancer Genes?
ICARE Social Media Post October 2023
What Are Established Cancer Genes?
There are many different inherited breast cancer genes, and different genes lead to different levels of breast cancer risk:
- ATM, BARD1, BRCA1, BRCA2, Breast Cancer, Cancer Risks, CDH1, CHEK2, PALB2, PTEN, RAD51C, RAD51D, Social Media, TP53
Permanent link to this article: https://inheritedcancer.net/post100423/
ICARE Newsletter Spring 2022
Ask the Expert
ICARE Newsletter Spring 2022
Ask the Expert
The below question was addressed by ICARE Founder, Dr. Tuya Pal, and her oncology colleague, Dr. Sonya Reid. Dr. Pal is a Professor of Genetic Medicine, Ingram Professor of Cancer Research, and the Associate Director for Cancer Health Disparities at VanderbiltIngram Cancer Center. Dr. Reid is an Assistant Professor of Hematology/Oncology at Vanderbilt University Medical …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2022-ask-the-expert/
ICARE Newsletter Fall 2022
Which Genes Are Confirmed as ‘Inherited Breast Cancer Genes’?
ICARE Newsletter Fall 2022
Which Genes Are Confirmed as ‘Inherited Breast Cancer Genes’?
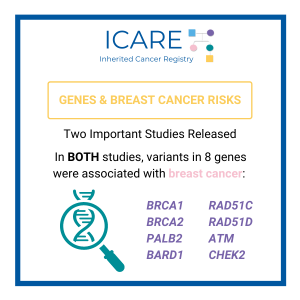
There were two large studies published early last year that evaluated which genes raise risks for breast cancer, including breast cancer patients from many centers worldwide, representing the largest available datasets to look at this question. These efforts were led by the worldwide Breast Cancer Association Consortium (BCAC)1 and the United States-based CARRIERS consortium. The …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2022-which-genes-are-confirmed-as-inherited-breast-cancer-genes/
ICARE Newsletter Fall 2021
Ask the Expert
ICARE Newsletter Fall 2021
Ask the Expert
In each newsletter, we give participants the opportunity to have their questions addressed by experts in thefield. This question was addressed by Kerry Schaffer, MD, medical oncologist at Vanderbilt University MedicalCenter with a focus on urological cancers. Q. Is there enough information to consider using PARP inhibitors to treat inherited forms ofprostate cancer? A. Over …
- ATM, BARD1, BRCA1, BRCA2, CHEK2, PALB2, PARP Inhibitors, Prostate Cancer, RAD51C, RAD51D
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2021-ask-the-expert/
ICARE Social Media Post February 2022
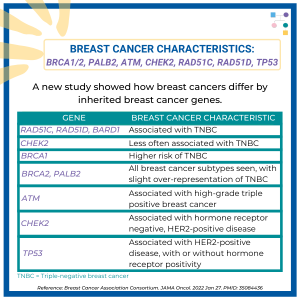
Breast Cancer Characteristics Across Genes
ICARE Social Media Post February 2022
Breast Cancer Characteristics Across Genes
A new study demonstrated that breast cancer pathology and other clinical features differ by inherited breast cancer gene. Read the full article to learn more!https://jamanetwork.com/journals/jamaoncology/fullarticle/2788577Reference: Breast Cancer Association Consortium. JAMA Oncol. 2022 Jan 27. PMID: 35084436
Permanent link to this article: https://inheritedcancer.net/post20122/
ICARE Social Media Post August 2021
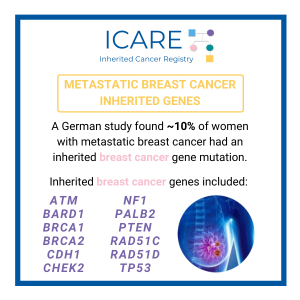
Metastatic Breast Cancer Genes
ICARE Social Media Post August 2021
Metastatic Breast Cancer Genes
For additional information, read the Journal of Clinical Oncology article at the link below 👇 https://ascopubs.org/doi/10.1200/JCO.20.01200
Permanent link to this article: https://inheritedcancer.net/post82021/
ICARE Social Media Post August 2021
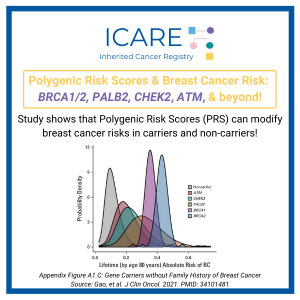
Breast Cancer Risk Among Breast Cancer Gene Carriers Varies by Polygenic Risk Score
ICARE Social Media Post August 2021
Breast Cancer Risk Among Breast Cancer Gene Carriers Varies by Polygenic Risk Score
For more information, read the 𝘑𝘰𝘶𝘳𝘯𝘢𝘭 𝘰𝘧 𝘊𝘭𝘪𝘯𝘪𝘤𝘢𝘭 𝘖𝘯𝘤𝘰𝘭𝘰𝘨𝘺 article at the link below:https://ascopubs.org/doi/figure/10.1200/JCO.20.01992
Permanent link to this article: https://inheritedcancer.net/post81321/
ICARE Social Media Post June 2021
A Population-Based Study of Genes Previously Implicated in Breast Cancer
ICARE Social Media Post June 2021
A Population-Based Study of Genes Previously Implicated in Breast Cancer
For additional information, read the article at the following link: https://www.nejm.org/doi/10.1056/NEJMoa2005936
- ATM, BARD1, BRCA1, BRCA2, Breast Cancer, Cancer Risks, CDH1, CHEK2, MSH6, PALB2, RAD51C, RAD51D, Social Media
Permanent link to this article: https://inheritedcancer.net/post62521/
ICARE Social Media Post March 2021
Three Articles: Breast Cancer Risks
ICARE Social Media Post March 2021
Three Articles: Breast Cancer Risks
For additional information about the: 》US-based study, visit: https://www.nejm.org/doi/full/10.1056/nejmoa2005936 》International study, visit: https://www.nejm.org/doi/full/10.1056/nejmoa1913948 》Accompanying editorial: https://www.nejm.org/doi/full/10.1056/NEJMe2035083
- ATM, BARD1, BRCA1, BRCA2, Breast Cancer, Cancer Risks, CDH1, CHEK2, MSH6, PALB2, RAD51C, RAD51D, Social Media
Permanent link to this article: https://inheritedcancer.net/post30921/
ICARE Newsletter Winter 2021
Inherited Breast Cancer Genes: Two New Important Articles Just Released
ICARE Newsletter Winter 2021
Inherited Breast Cancer Genes: Two New Important Articles Just Released
Results of a United States (U.S.)-based study1 and an international study2 were released in January in the New England Journal of Medicine and provide a much clearer picture about the role of inherited breast cancer genes in women without a family history of cancer, and how common these genes may be in the general population. …
- ATM, BARD1, BRCA1, BRCA2, Breast Cancer, Cancer Risks, CHEK2, PALB2, RAD51C, RAD51D
Permanent link to this article: https://inheritedcancer.net/2nlw2021/
ICARE Newsletter Winter 2021
Updates to National Comprehensive Cancer Network (NCCN) Guidelines Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic
ICARE Newsletter Winter 2021
Updates to National Comprehensive Cancer Network (NCCN) Guidelines Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic
Released September 8th, 2020: Genetic testing criteria by cancer type: Breast Cancer: Broadened to include relatives with ALL grades of prostate cancer (not just high-grade) Having multiple breast cancer diagnoses no longer depends on whether the diagnoses were on two different breasts Prostate Cancer: Now includes cribriform histology and ANY risk group (not just high-grade …
Permanent link to this article: https://inheritedcancer.net/1nlw2021/
ICARE Featured Video September & October 2020
NCCN Genetic/Familial Breast, Ovarian, and Pancreatic Guidelines
ICARE Featured Video September & October 2020
NCCN Genetic/Familial Breast, Ovarian, and Pancreatic Guidelines
- By admin in Case Conference Videos
Below you may watch a featured video from the September 2020 and October 2020 Genetics Case Conference, which outlined updates to the National Comprehensive Cancer Network (NCCN) guidelines. Check out the full guidelines by creating a FREE account at: https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf
Permanent link to this article: https://inheritedcancer.net/video100820/
ICARE Social Media Post September 2020
NCCN Breast Cancer Risk Management Updates by Gene
ICARE Social Media Post September 2020
NCCN Breast Cancer Risk Management Updates by Gene
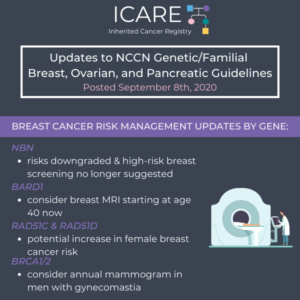
The National Comprehensive Cancer Network (NCCN) released new guidelines on September 8th, 2020, which included updates to breast cancer risk management recommendations by gene as follows: NBN – high-risk breast screening was removed as there is insufficient evidence to support high cancer risks BARD1 – added consideration for high-risk breast screening starting at age 40 …
Permanent link to this article: https://inheritedcancer.net/post91020/
ICARE Social Media Post May 2020
Platinum Based Chemotherapy for Metastatic Pancreatic Cancer
ICARE Social Media Post May 2020
Platinum Based Chemotherapy for Metastatic Pancreatic Cancer
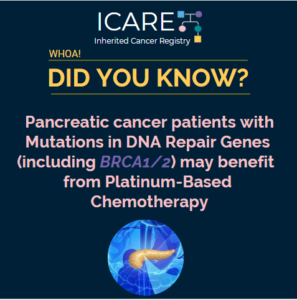
A recent study found that patients with metastatic pancreatic cancer who had mutations in the DNA repair genes (either inherited or just in the tumor) had better clinical outcomes after platinum-based chemotherapy compared to patients without these mutations. Check out the link to full article: https://clincancerres.aacrjournals.org/content/early/2020/05/20/1078-0432.CCR-20-0418
Permanent link to this article: https://inheritedcancer.net/post52920/
ICARE Newsletter Summer 2018
Updates to NCCN Genetic/Familial High-Risk Assessment: Breast and Ovarian Guidelines
ICARE Newsletter Summer 2018
Updates to NCCN Genetic/Familial High-Risk Assessment: Breast and Ovarian Guidelines
(Version 1.2019, posted July 11, 2018) Regardless of family history, some individuals with a hereditary breast- and ovarian-related cancer may benefit from genetic testing to determine eligibility for targeted treatment The multi-gene testing section table was updated with: A potential association of ATM with ovarian cancer risk Potential increased risk of BARD1 with breast cancer …
Permanent link to this article: https://inheritedcancer.net/1nls2018/
ICARE Newsletter Summer 2017
Breast and Ovarian Cancer Associations for Genes Tested Through Multi-Gene Panels
ICARE Newsletter Summer 2017
Breast and Ovarian Cancer Associations for Genes Tested Through Multi-Gene Panels
As testing for multiple genes at the same time (“multi-gene panel testing”) has become increasingly available with tremendous advances in genetic testing technology, it has become critical to evaluate and refine cancer associations and levels of risk for many of these genes now tested. Through a commercial laboratory database of almost 100,000 results of multi-gene …
Permanent link to this article: https://inheritedcancer.net/2nls2017/
ICARE Newsletter Summer 2015
2015 NCCN Clinical Practice Guideline Update
ICARE Newsletter Summer 2015
2015 NCCN Clinical Practice Guideline Update
Breast and Ovarian Management Based on Genetic Test Resultsa Recommend Breast MRIc (>20% lifetime risk of breast cancerd) Recommend Risk-reducing salpingo-oophorectomy Discuss Option of Risk-reducing mastectomy Intervention warranted based on gene and/or risk level ATM, BRCA1, BRCA2, CDH1, CHEK2, PALB2, PTEN, STK11, TP53 BRCA1, BRCA2, Lynch syndromee BRCA1, BRCA2, CDH1, PTEN, TP53 Insufficient evidence …
- ATM, BARD1, BRCA1, BRCA2, Breast Cancer, BRIP1, CDH1, CHEK2, Guideline/Policy Updates, Lynch Syndrome, Ovarian Cancer, PALB2, PTEN, RAD51C, RAD51D, STK11, TP53
Permanent link to this article: https://inheritedcancer.net/1nls2015/