In a new study of almost 70,000 women, including those with hereditary forms of breast cancer, breast cancer risk was higher in those with a family history of breast cancer. Family history raised breast cancer risks: ✨Why is this important? ✨This type of information to personalize risk estimates can help women to guide screening and …
Tag: Cancer Prevention
Permanent link to this article: https://inheritedcancer.net/post10282025/
ICARE Social Media Post October 2025
NCCN CEG Guidelines (V1.2025): TP53 Post
ICARE Social Media Post October 2025
NCCN CEG Guidelines (V1.2025): TP53 Post
The National Comprehensive Cancer Network (NCCN) just released updated Genetic/Familial High-Risk Assessment: Colorectal, Endometrial, Gastric Cancer guidelines on June 13th, 2025 (Version 1.2025). Included in these new guidelines are updates to the TP53 content as follows: To read more, check out the full guidelines by creating a FREE account at:https://www.nccn.org/professionals/physician_gls/pdf/genetics_ceg.pdf
Permanent link to this article: https://inheritedcancer.net/post10062025/
ICARE Newsletter Fall 2025
Lynch Syndrome: Showing the Importance of Family Testing
ICARE Newsletter Fall 2025
Lynch Syndrome: Showing the Importance of Family Testing
Cascade testing refers to testing family members for a gene mutation after another family member is found to have a mutation. Once family members get cascade testing, they can also benefit from screening, cancer prevention, and early detection strategies. A study conducted a microsimulation model to look at the cost effectiveness of cascade testing of …
Permanent link to this article: https://inheritedcancer.net/nlf20258/
Permanent link to this article: https://inheritedcancer.net/post09162025/
Permanent link to this article: https://inheritedcancer.net/post08262025/
ICARE Social Media Post June 2025
Lynch Syndrome: Showing the Importance of Family Testing
ICARE Social Media Post June 2025
Lynch Syndrome: Showing the Importance of Family Testing
Cascade testing refers to testing “at-risk” family members for a gene mutation, once the mutation has been found in a family member. For Lynch syndrome, once family members get cascade testing, they can also benefit from screening, cancer prevention, and early detection strategies. Findings of a microsimulation model looking at the cost effectiveness of cascade …
Permanent link to this article: https://inheritedcancer.net/post061025/
ICARE Social Media Post May 2025
Strategy to Intercept Cancers in Lynch Syndrome
ICARE Social Media Post May 2025
Strategy to Intercept Cancers in Lynch Syndrome
Lynch syndrome related cancers have a distinct immune profile. They are generally “immunogenic”, meaning there might be opportunities to develop immune-interception strategies to prevent cancer. What is cancer “interception”? A new study based on work in mice showed that an EZH2 inhibitor (called GSK503) given over 9 weeks in mice lowered the number of adenomas …
Permanent link to this article: https://inheritedcancer.net/post50525/
ICARE Featured Video April 2025
Increasing Cascade Testing: ConnectMyVariant
ICARE Featured Video April 2025
Increasing Cascade Testing: ConnectMyVariant
Below is a featured video from the April 2025 case conference, during which Brian Shirts, MD, PhD from Vanderbilt University Medical Center and Rachel Pearlman, MS, CGC from The Ohio State University highlight how connecting with relatives through publicly available platforms like ConnectMyVariant can lead to increased cascade testing
Permanent link to this article: https://inheritedcancer.net/video41025/
ICARE Newsletter Fall 2024
BRCA1/2 Carriers and Pregnancy-Related Risk
ICARE Newsletter Fall 2024
BRCA1/2 Carriers and Pregnancy-Related Risk
A recent study reported that pregnancy after breast cancer was safe for both mother and baby.1 Specifically, the researchers found that pregnancy after breast cancer was not associated with adverse maternal prognosis or fetal outcomes. Another study reported that breast cancer after pregnancy could be associated with poorer outcomes. Specifically, breast cancer diagnosed within 10 …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2024-brca1-2-carriers-and-pregnancy-related-ris/
ICARE Social Media Post August 2024
How to Access NCCN Guidelines
ICARE Social Media Post August 2024
How to Access NCCN Guidelines
Unlock valuable resources for your health journey! 🌟 The National Comprehensive Cancer Network (NCCN) guides clinical practice by providing free access to their guidelines that are available to both patients and healthcare providers. To access the most recent version of cancer-related guidelines that are updated at least annually, create a free username and password at …
Permanent link to this article: https://inheritedcancer.net/post82024/
ICARE Social Media Post February 2024 Updates to NCCN Guidelines: Genetic/Familial Breast, Ovarian, and Pancreatic Post #2
The National Comprehensive Cancer Network (NCCN) just released updated Genetic/Familial Breast, Ovarian, and Pancreatic Cancer guidelines on February 12th, 2024! Updates include expanded guidance about gynecologic cancers in BRCA1 and BRCA2, including:✓ Reproductive considerations✓ Non-surgical and surgical risk reduction✓ Salpingectomy✓ Hysterectomy considerations✓ HRT after risk-reducing removal of the ovaries You can check out the full …
Permanent link to this article: https://inheritedcancer.net/post21324_2/
ICARE Social Media Post February 2024
Updates to NCCN Guidelines: Genetic/Familial Breast, Ovarian, and Pancreatic Post #1
ICARE Social Media Post February 2024
Updates to NCCN Guidelines: Genetic/Familial Breast, Ovarian, and Pancreatic Post #1
The National Comprehensive Cancer Network (NCCN) just released updated Genetic/Familial Breast, Ovarian, and Pancreatic Cancer guidelines on February 12th, 2024! Updates include adding contralateral breast cancer risks for BRCA1, BRCA2, PALB2, CHEK2, and other genes to the GENE-A (Cancer Risk Management) table 🧬 You can check out the full guidelines by creating a FREE account …
Permanent link to this article: https://inheritedcancer.net/post21324/
ICARE Featured Video January 2024
Transplant Eligibility and Inherited Cancer Results
ICARE Featured Video January 2024
Transplant Eligibility and Inherited Cancer Results
Below is a featured video from the January 2024 case conference, during which Katie Lang, MS, CGC discusses transplant eligibility in the context of inherited cancer genetic test results.
Permanent link to this article: https://inheritedcancer.net/video11224/
ICARE Featured Video December 2023
Care for Gender Diverse Individuals with or at Risk for Inherited Cancer Predisposition
ICARE Featured Video December 2023
Care for Gender Diverse Individuals with or at Risk for Inherited Cancer Predisposition
Below is a featured video from the December 2023 case conference, during which Kim Zayhowski, MS, CGC, Sarah Roth, ScM, and Sasha Weiss, MA discuss care for gender diverse individuals with or at risk for inherited cancer predisposition. Please visit our YouTube channel to watch the full December conference, including valuable discussion from genetics professionals!
Permanent link to this article: https://inheritedcancer.net/video121423/
ICARE Featured Video November 2023
Pancreatic Cancer Screening
ICARE Featured Video November 2023
Pancreatic Cancer Screening
Below is a featured video from the November 2023 case conference, during which Bryson Katona, MD, PhD from Penn Medicine presents on pancreatic cancer screening. Please visit our YouTube channel to watch the full November conference, including valuable discussion from genetics professionals!
Permanent link to this article: https://inheritedcancer.net/video110923/
ICARE Featured Video October 2023
Management of Individuals with CHEK2 Mutations
ICARE Featured Video October 2023
Management of Individuals with CHEK2 Mutations
Below is a featured video from the October 2023 case conference, during which Helen Hanson, MD from the Royal Devon University Healthcare NHS Foundation Trust and Douglas Stewart, MD from the National Cancer Institute present on the management of individuals with CHEK2 germline pathogenic variants. Please visit our YouTube channel to watch the full October …
Permanent link to this article: https://inheritedcancer.net/video101223/
ICARE Newsletter Fall 2023
Newly released ACMG Clinical Practice Resource on CHEK2 Developed Through a Group of Worldwide Experts!
ICARE Newsletter Fall 2023
Newly released ACMG Clinical Practice Resource on CHEK2 Developed Through a Group of Worldwide Experts!
A person with a pathogenic variant in the CHEK2 gene may be at an increased risk for developing breast and other cancers. This ACMG Clinical Practice Resource, published in ACMG’s flagship journal, Genetics in Medicine, provides valuable information for healthcare providers caring for individuals with pathogenic variants in the CHEK2gene. This new ACMG Practice Resource …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2023-newly-released-acmg-clinical-practice-resource-on-chek2-developed-through-a-group-of-worldwide-experts/
ICARE Newsletter Fall 2023
Tamoxifen and Breast Cancer Risk in Women with a BRCA1 or BRCA2 Mutation
ICARE Newsletter Fall 2023
Tamoxifen and Breast Cancer Risk in Women with a BRCA1 or BRCA2 Mutation
In a study of female BRCA carriers, including ICARE participants, those that used tamoxifen and/or raloxifenewere compared to those that did not. After an average follow-up time of almost 7 years, 10.9% of those in the tamoxifen/raloxifene group were diagnosed with breast cancer, compared to 14.3% in the group that did not use thesedrugs. This …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2023-tamoxifen-and-breast-cancer-risk-in-women-with-a-brca1-or-brca2-mutation/
ICARE Newsletter Fall 2023
Recommendations for Inherited Ovarian Cancer Genes: United Kingdom (UK)
ICARE Newsletter Fall 2023
Recommendations for Inherited Ovarian Cancer Genes: United Kingdom (UK)
Through consensus, management recommendations were developed in the UK for females with pathogenic variants inthe following inherited ovarian cancer genes: RAD51C, RAD51D, BRIP1, and PALB2. Hanson, et al. J Med Genet. 2023;60(5):417-429. PMID: 36411032. Social media post September 26th, 2023.Available at https://tinyurl.com/post9262023.
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2023-recommendations-for-inherited-ovarian-cancer-genes-united-kingdom-uk/
ICARE Newsletter Fall 2023
Mammograms: New U.S. Preventive Services Task Force (USPSTF) Recommendations
ICARE Newsletter Fall 2023
Mammograms: New U.S. Preventive Services Task Force (USPSTF) Recommendations
On May 9th, 2023, the USPSTF published a new draft recommendation for all cisgender women and those assigned female sex at birth to do mammograms from ages 40 to 74, every two years, for those at average (population) risk forbreast cancer. This change in recommendation is due to recent troubling trends, including an increase in …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2023-mammograms-new-u-s-preventive-services-task-force-uspstf-recommendations/
ICARE Newsletter Fall 2023
National Comprehensive Cancer Network (NCCN) Guidelines Updates
ICARE Newsletter Fall 2023
National Comprehensive Cancer Network (NCCN) Guidelines Updates
Check out the full NCCN guidelines by creating a FREE account at www.nccn.org Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic CancerReleased August 28th, 2023 (V1.2024) › Transgender, Non-Binary, and Gender Diverse Individuals: NEW section on care (Page 63-66, TNBGD-1 to 4)› Li-Fraumeni Syndrome: Significant updates to content (risks and care) (Pages 57-60, LIFR-A): Table added …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2023-national-comprehensive-cancer-network-nccn-guidelines-updates/
ICARE Social Media Post August 2023
New Article – Cancer Risk Management
ICARE Social Media Post August 2023
New Article – Cancer Risk Management
A recent study, which 𝗶𝗻𝗰𝗹𝘂𝗱𝗲𝗱 𝗜𝗖𝗔𝗥𝗘 𝗽𝗮𝗿𝘁𝗶𝗰𝗶𝗽𝗮𝗻𝘁𝘀, found that getting care according to guidelines depends on: Therefore, we need ways to improve knowledge among healthcare providers and trust in care among patients! Use this link to learn more: https://tinyurl.com/n26m4zys Reference: Dean, et al. Genet Med. 2023;100945. PMID: 37515473.
Permanent link to this article: https://inheritedcancer.net/post80723/
ICARE Social Media Post May 2023
Women’s Health Week
ICARE Social Media Post May 2023
Women’s Health Week
In honor of National Women’s Health Week from May 14th to May 20th, we would like to encourage you to speak with your healthcare provider to make sure you are up-to-date on your cancer screenings, such as mammograms and colonoscopies, to help prevent cancer or find it early when it is easier to treat. Spread …
Permanent link to this article: https://inheritedcancer.net/post51523/
ICARE Featured Video February 2023
CanRisk: Personalizing Cancer Risk Prediction
ICARE Featured Video February 2023
CanRisk: Personalizing Cancer Risk Prediction
Below you may watch a featured video from the February 2023 Genetics Case Conference, which focused on CanRisk, a tool to estimate future breast and ovarian cancer risks, with guest expert Antonis Antoniou, PhD from the University of Cambridge.
Permanent link to this article: https://inheritedcancer.net/video21023/
ICARE Social Media Post August 2022
Nipple sparing mastectomy: BRCA1/2 carriers
ICARE Social Media Post August 2022
Nipple sparing mastectomy: BRCA1/2 carriers
According to a recent study, a bilateral prophylactic nipple-sparing mastectomy appears to be at least equally as safe as other types of mastectomy for preventing breast cancer. Read the full article at the link: https://pubmed.ncbi.nlm.nih.gov/34342702/Reference: Stanek et al. Aesthetic Plast Surg. 2022 Apr;46(2):706-711. PMID: 34342702.
Permanent link to this article: https://inheritedcancer.net/post82922/
ICARE Social Media Post February 2022
BRCA1/2 Cancer Risks: Oral Contraceptives
ICARE Social Media Post February 2022
BRCA1/2 Cancer Risks: Oral Contraceptives
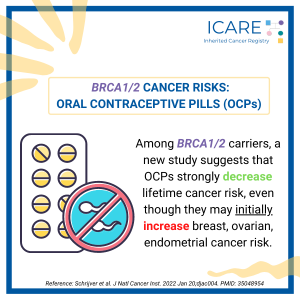
A new study found that among BRCA1/2 carriers oral contraceptive use strongly decreases lifetime cancer risk, despite an 𝗶𝗻𝗶𝘁𝗶𝗮𝗹 increase in breast, ovarian, and endometrial cancer risk. Read the full article to learn more!https://pubmed.ncbi.nlm.nih.gov/35048954/Reference: Schrijver et al. J Natl Cancer Inst. 2022 Jan 20;djac004. PMID: 35048954
Permanent link to this article: https://inheritedcancer.net/post21522/
ICARE Featured Video August 2021
Updates to National Comprehensive Cancer Network (NCCN) Guidelines
ICARE Featured Video August 2021
Updates to National Comprehensive Cancer Network (NCCN) Guidelines
Below you may watch a featured video from the August 2021 ICARE Genetics Case Conferences outlining updates to National Comprehensive Cancer Network (NCCN) guidelines.
Permanent link to this article: https://inheritedcancer.net/video81221_2/
Permanent link to this article: https://inheritedcancer.net/post72021/
Permanent link to this article: https://inheritedcancer.net/video31121/
Permanent link to this article: https://inheritedcancer.net/1nlw2021/
ICARE Newsletter Winter 2021
Learning You Have a Mutation in an Inherited Cancer Gene: What’s Next?
ICARE Newsletter Winter 2021
Learning You Have a Mutation in an Inherited Cancer Gene: What’s Next?
The benefits achieved through genetic testing for inherited cancer only happen by acting upon the results. This can be through guiding cancer treatment, receiving appropriate cancer risk management strategies, and sharing results with at-risk family members so they too can benefit from this information. We recently reported on results of our study, made possible through …
Permanent link to this article: https://inheritedcancer.net/6nlw2021/
ICARE Publication January 2021
Qualitative Methods for Refining a Web-Based Educational Tool for Patients Focused on Inherited Cancer Predisposition
ICARE Publication January 2021
Qualitative Methods for Refining a Web-Based Educational Tool for Patients Focused on Inherited Cancer Predisposition
Abstract To address the increasing demand for inherited cancer genetic testing, we developed and evaluated a web-based educational tool to streamline genetic counseling (GC). Consented patients viewed the initial prototype containing core content (Version 1-Core) and provided feedback through three open-ended survey questions. Additional data were collected through individual interviews from a subgroup who viewed …
Permanent link to this article: https://inheritedcancer.net/pub10521_2/
ICARE Social Media Post October 2020
CDH1: Cancer Risks and Risk Management
ICARE Social Media Post October 2020
CDH1: Cancer Risks and Risk Management
Gene: 𝘾𝘿𝙃𝟭 Cancer Risks and Management per NCCN Genetic/Familial High-Risk Assessment: Breast/Ovarian/Pancreatic Version 1.2021 and Gastric Version 3.2020: 𝗪𝗼𝗺𝗲𝗻: Breast cancer risk: Elevated at 55% – Recommend annual mammogram starting at age 30; consider breast MRI with contrast starting at age 30. 𝗠𝗲𝗻 𝗮𝗻𝗱 𝗪𝗼𝗺𝗲𝗻: Stomach cancer risk: Elevated at 83% for women, and 67% …
Permanent link to this article: https://inheritedcancer.net/post103020/
ICARE Social Media Post October 2020
PTEN: Cancer Risks and Risk Management
ICARE Social Media Post October 2020
PTEN: Cancer Risks and Risk Management
Gene: 𝙋𝙏𝙀𝙉 Cancer Risks and Management per National Comprehensive Cancer Network (NCCN) Genetic/Familial High-Risk Assessment: Breast/Ovarian/Pancreatic Version 1.2021: 𝗪𝗼𝗺𝗲𝗻: Breast cancer risk: Elevated at 85% – Recommend annual mammogram starting at age 30-35 (or 5-10 years before the earliest known breast cancer in the family); consider breast MRI with contrast starting at age 30-35; consider …
Permanent link to this article: https://inheritedcancer.net/post102320/
ICARE Social Media Post October 2020
TP53: Cancer Risks and Risk Management
ICARE Social Media Post October 2020
TP53: Cancer Risks and Risk Management
Gene: 𝙏𝙋𝟱𝟯 Syndrome: Li-Fraumeni Cancer Risks and Management per National Comprehensive Cancer Network (NCCN) Genetic/Familial High-Risk Assessment: Breast/Ovarian/Pancreatic Version 1.2021: 𝗪𝗼𝗺𝗲𝗻: Breast cancer risk: Elevated at 54% – Recommend clinical breast exam every 6-12 months starting at age 20, annual breast MRI with contrast starting at age 20, and annual mammogram starting at age 30; …
Permanent link to this article: https://inheritedcancer.net/post101620/
ICARE Social Media Post October 2020
CHEK2: Cancer Risks and Risk Management
ICARE Social Media Post October 2020
CHEK2: Cancer Risks and Risk Management
Gene: 𝘾𝙃𝙀𝙆2 Cancer Risks and Management per National Comprehensive Cancer Network (NCCN) Genetic/Familial High-Risk Assessment: Colorectal Version 1.2020 & Breast/Ovarian/Pancreatic Version 1.2021: 𝗪𝗼𝗺𝗲𝗻: Breast Cancer Risk: Elevated at 28-44% – Recommend annual mammogram starting at age 40 and consider annual breast MRIs with contrast starting at age 40. 𝗠𝗲𝗻 𝗮𝗻𝗱 𝗪𝗼𝗺𝗲𝗻: Colorectal Cancer Risk: Elevated …
Permanent link to this article: https://inheritedcancer.net/post100920/
Permanent link to this article: https://inheritedcancer.net/video100820/
ICARE Social Media Post October 2020
ATM: Cancer Risks and Risk Management
ICARE Social Media Post October 2020
ATM: Cancer Risks and Risk Management
Gene: 𝘼𝙏𝙈 Cancer Risks and Management (per NCCN Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic Version 1.2021) 𝗪𝗼𝗺𝗲𝗻: Breast cancer risk: Elevated at 30% – Recommend annual mammogram starting at age 40 and consider annual breast MRIs starting at age 40. Ovarian cancer risk: Possibly increased, not well established – Manage based on family history. …
Permanent link to this article: https://inheritedcancer.net/post100220/
ICARE Social Media Post August 2020
Inherited Prostate Cancer Risk
ICARE Social Media Post August 2020
Inherited Prostate Cancer Risk
Over 600,000 men age 40 and older who were part of a family with at least three consecutive generations affected with prostate cancer were studied from the Utah Population Database. Findings from this study showed that: 36,000 had prostate cancer (5.9%) 2,500 had early-onset disease (7%) 4,000 had lethal disease (11.1%) 15,000 had clinically significant …
Permanent link to this article: https://inheritedcancer.net/post81420/
ICARE Publication May 2020
Cancer risk management among female BRCA1/2, PALB2, CHEK2, and ATM carriers
ICARE Publication May 2020
Cancer risk management among female BRCA1/2, PALB2, CHEK2, and ATM carriers
Abstract Purpose: Identification of inherited breast cancer may guide cancer risk management. We sought to compare risk management practices across women with inherited breast cancer genes. Methods: Females with a pathogenic/likely pathogenic (P/LP) variant in BRCA1/2, PALB2, CHEK2, and/or ATM were surveyed about cancer risk management. Comparisons were made across genes. Results: The 235 participants with P/LP variants …
Permanent link to this article: https://inheritedcancer.net/pub52220/
ICARE Newsletter Winter 2020
Community Spotlight
ICARE Newsletter Winter 2020
Community Spotlight
I was diagnosed with breast cancer in December 2018 and was found to be PALB2+. The PALB2 gene had not been tested for when my older sister was diagnosed with breast cancer and had genetic testing done four years earlier. This was new! My cancer was very similar to my sister’s, but being PALB2+ changed …
Permanent link to this article: https://inheritedcancer.net/spotlightnlw2020/
ICARE Social Media Post February 2020
Medication Use for Risk Reduction
ICARE Social Media Post February 2020
Medication Use for Risk Reduction
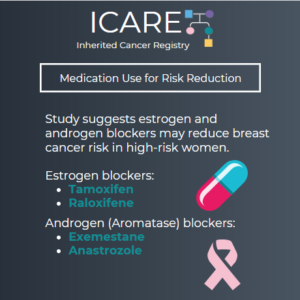
Based on studies that show benefits of estrogen and androgen blockers in reducing breast cancer risk in high-risk women, the United States Preventative Service Task Force (USPSTF) recommends that clinicians offer risk-reducing medications to women at increased risk for breast cancer; however, they recommend against routine use of these medications in average-risk women. Hormone receptor …
Permanent link to this article: https://inheritedcancer.net/post21120/
ICARE Social Media Post January 2020
Ovarian Cancer Screening in BRCA1 Carriers
ICARE Social Media Post January 2020
Ovarian Cancer Screening in BRCA1 Carriers
A new study of Polish BRCA1 carriers showed that screening through transvaginal ultrasound was not effective or reliable in detecting ovarian cancer early. Consequently, preventive oophorectomy remains the only proven method to lower ovarian cancer risks and increase survival. Check out the article at: https://www.ncbi.nlm.nih.gov/pubmed/31500890. Check out the NCCN ovarian cancer screening recommendations for BRCA1 …
Permanent link to this article: https://inheritedcancer.net/post11720/
ICARE Social Media Post January 2020
Polygenic Risk Scores
ICARE Social Media Post January 2020
Polygenic Risk Scores
A Polygenic Risk Score (PRS) is calculated using genetic differences throughout someone’s DNA, in combination with clinical and family history of cancer. This unique type of score may be utilized to calculate the lifetime risk of breast cancer. Important facts about PRS’s: Different from BRCA1/2 testing, which look for changes in these specific genes Should …
Permanent link to this article: https://inheritedcancer.net/post11420/
ICARE Featured Video December 2019
NCCN Genetic/Familial Breast, Ovarian, and Pancreatic Guidelines
ICARE Featured Video December 2019
NCCN Genetic/Familial Breast, Ovarian, and Pancreatic Guidelines
Below you may watch a featured video from the December 2019 Genetics Case Conference, which outlined updates to the National Comprehensive Cancer Network (NCCN) guidelines.
Permanent link to this article: https://inheritedcancer.net/video121219/
ICARE Publication April 2019
International trends in the uptake of cancer risk reduction strategies in women with a BRCA1 or BRCA2 mutation
ICARE Publication April 2019
International trends in the uptake of cancer risk reduction strategies in women with a BRCA1 or BRCA2 mutation
Abstract Background: Women with a BRCA1 or BRCA2 mutation face high risks of breast and ovarian cancer. In the current study, we report on uptake of cancer screening and risk-reduction options in a cohort of BRCA mutation carriers from ten countries over two time periods (1995 to 2008 and 2009 to 2017). Methods: Eligible subjects were identified …
Permanent link to this article: https://inheritedcancer.net/pub41119/
ICARE Publication November 2018
Health beliefs associated with readiness for genetic counseling among high risk breast cancer survivors
ICARE Publication November 2018
Health beliefs associated with readiness for genetic counseling among high risk breast cancer survivors
Abstract We used the Health Belief Model (HBM) to explore factors associated with readiness for genetic counseling among breast cancer survivors. Breast cancer survivors meeting NCCN genetic counseling referral criteria completed questionnaires capturing demographic and clinical information and factors guided by the HBM, including health beliefs, psychosocial variables, and cues to action. Using logistic regression, …
Permanent link to this article: https://inheritedcancer.net/pub112818/
ICARE Newsletter Summer 2018
Prevention of Colorectal Cancer Among Individuals with Familial Adenomatous Polyposis (FAP)
ICARE Newsletter Summer 2018
Prevention of Colorectal Cancer Among Individuals with Familial Adenomatous Polyposis (FAP)
Through a randomized trial, patients with FAP were treated with sulindac and erlotinib versus placebo for 6 months. Results of the study showed that those treated with sulindac and erlotinib had 70% fewer polyps than those in the placebo group. The lower number of polyps was seen in both those with an intact colorectum and …
Permanent link to this article: https://inheritedcancer.net/9nls2018/
ICARE Newsletter Summer 2018
Ask the Expert
ICARE Newsletter Summer 2018
Ask the Expert
The following question was addressed by Ronald D. Alvarez, MD, MBA who is Professor, Chairman, and Clinical Service Chief of the Department of Obstetrics and Gynecology at Vanderbilt University Medical Center in Nashville, Tennessee. Dr. Alvarez has been the recipient of several National Cancer Institute (NCI) and other industry funded grants in support of his …
Permanent link to this article: https://inheritedcancer.net/10nls2018/
ICARE Newsletter Summer 2016
Cancer Chemoprevention in Individuals with Familial Adenomatous Polyposis (FAP)
ICARE Newsletter Summer 2016
Cancer Chemoprevention in Individuals with Familial Adenomatous Polyposis (FAP)
Prior research has demonstrated that NSAIDs significantly reduce colonic and rectal polyp burden among individuals with FAP although their impact on outcomes remains to be determined.1,2 Recent data extended these results to the small intestine through completion of a randomized clinical trial among patients with FAP which demonstrated that use of sulindac and erlotinib compared …
Permanent link to this article: https://inheritedcancer.net/7nls2016/
ICARE Newsletter Winter 2016
The Importance of Sharing Genetic Test Results with Family Members
ICARE Newsletter Winter 2016
The Importance of Sharing Genetic Test Results with Family Members
Once an individual has had genetic testing for inherited cancer predisposition this information could help their close family members. For example, when a BRCA mutation or a mutation in another inherited cancer gene is found, it is important for close family members (with or without a diagnosis of cancer) to know so they too can …
Permanent link to this article: https://inheritedcancer.net/1nlw2016/
ICARE Newsletter Summer 2015
Advances in Preventive and Treatment Approaches for Individuals with Lynch Syndrome
ICARE Newsletter Summer 2015
Advances in Preventive and Treatment Approaches for Individuals with Lynch Syndrome
A study of over 1800 individuals with a mutation in one of the Lynch Syndrome genes was recently completed to assess whether aspirin and ibuprofen use may reduce colon cancer risk. Results showed that in those who took aspirin or ibuprofen for between 1 month and 4.9 years, the colon cancer risks were lower than …
Permanent link to this article: https://inheritedcancer.net/6nls2015/
ICARE Newsletter Winter 2015
Ask the Expert
ICARE Newsletter Winter 2015
Ask the Expert
The following question was addressed to Dr. Steven Narod who is a Tier I Canada Research Chair in Breast Cancer and a senior scientist at Women’s College Research Institute in Toronto, Canada. Dr. Narod is a world-leader in the field of breast and ovarian cancer genetics. Over the course of his career, he has profoundly shaped …
Permanent link to this article: https://inheritedcancer.net/3nlw2015/
ICARE Newsletter Summer 2014
Is Breast Cancer Risk Affected by Timing of Oral Contraceptives in BRCA1 Carriers?
ICARE Newsletter Summer 2014
Is Breast Cancer Risk Affected by Timing of Oral Contraceptives in BRCA1 Carriers?
Although oral contraceptives (OC) reduce the risk of ovarian cancer in BRCA carriers,1 it is possible that they may raise breast cancer risk. Thus it is important to understand whether age at OC use is a factor when determining impact on breast cancer risk. To address this question, a recent study (which included data from …
Permanent link to this article: https://inheritedcancer.net/2nls2014/
ICARE Newsletter Summer 2014
New Study to Suggest Benefits of Oophorectomy in BRCA Mutation Carriers
ICARE Newsletter Summer 2014
New Study to Suggest Benefits of Oophorectomy in BRCA Mutation Carriers
A recent study published in the Journal of Clinical Oncology reported that prophylactic oophorectomy resulted in reducing the risk of ovarian cancer by 80%, and reduced all-cause mortality by 77%.1 The authors reported results on almost 5800 women, of whom 186 developed either ovarian, fallopian tube or peritoneal cancer. Women who had bilateral oophorectomy had …
Permanent link to this article: https://inheritedcancer.net/4nls2014/
ICARE Newsletter Winter 2014
Tamoxifen May Reduce Contralateral Breast Cancer Risk in BRCA Carriers
ICARE Newsletter Winter 2014
Tamoxifen May Reduce Contralateral Breast Cancer Risk in BRCA Carriers
There have been suggestions that Tamoxifen may reduce risks for contralateral breast cancer (i.e., breast cancer in the other breast) in BRCA carriers if taken after the initial breast cancer diagnosis, based mainly on retrospective studies. Only one prospective study has looked at this question, and showed that Tamoxifen may be useful in BRCA2, but …
Permanent link to this article: https://inheritedcancer.net/2nlw2014/
ICARE Newsletter Summer 2013
Oophorectomy Following Menopause
ICARE Newsletter Summer 2013
Oophorectomy Following Menopause
Prior studies have indicated that removal of the ovaries and fallopian tubes reduces the ovarian cancer risk by ~80% and breast cancer risk by ~50%, particularly when performed pre-menopausally. However a recent case control study of 2854 pairs of women with a BRCA1 or BRCA2 mutation with or without breast cancer showed that the risk …
Permanent link to this article: https://inheritedcancer.net/5nls2013/
ICARE Newsletter Winter 2013
Points to Consider Regarding Bilateral Salpingectomy as a Risk Reduction Procedure for Ovarian Cancer
ICARE Newsletter Winter 2013
Points to Consider Regarding Bilateral Salpingectomy as a Risk Reduction Procedure for Ovarian Cancer
Over the last few years, there has been evidence to suggest that a substantial proportion of ovarian cancer may start in the fallopian tubes, although some cancer clearly arises in the ovary. As a result, removal of both fallopian tubes (called ‘bilateral salpingectomy’) has been suggested as an interim procedure to reduce risk in BRCA …
Permanent link to this article: https://inheritedcancer.net/2nlw2013/
ICARE Newsletter Summer 2012
Ask the Expert: What are the recommendations for screening following prophylactic surgeries?
ICARE Newsletter Summer 2012
Ask the Expert: What are the recommendations for screening following prophylactic surgeries?
The following questions were addressed by Drs. Pal and Lancaster at the Moffitt Cancer Center: Q. What are the recommendations for screening following prophylactic surgeries? A. In BRCA mutation carriers, bilateral prophylactic mastectomy reduces the risk of breast cancer by over 90%. It essentially lowers the risk of breast cancer to below that of the …
Permanent link to this article: https://inheritedcancer.net/5nls2012/