In January 2024, the American Society of Clinical Oncology (ASCO) in conjunction with the Society of Surgical Oncology released new guidelines for germline testing in patients with breast cancer, which include the following: For a full list of recommendations in this guideline, the article is available at: https://ascopubs.org/doi/10.1200/JCO.23.02225 Bedrosian, et al. J Clin Oncol. 2024;42(5):584-604. PMID:38175972. Social …
Tag: STK11
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2024-new-guidelines-released-through-asco-society-of-oncology-germline-testing-in-patients-with-breast-center/
ICARE Newsletter Spring 2024
National Comprehensive Cancer Network (NCCN) Guideline Updates
ICARE Newsletter Spring 2024
National Comprehensive Cancer Network (NCCN) Guideline Updates
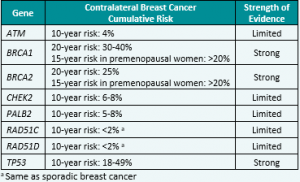
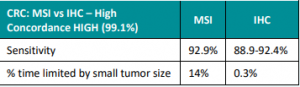
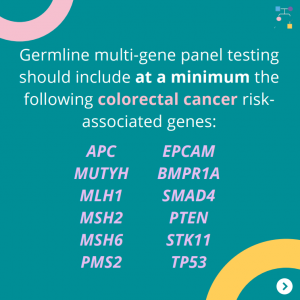
Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic Cancer – Released February 12th, 2024 (V3.2024) Check out the full guidelines by creating a FREE account at www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf Contralateral breast cancer risks in these updated guidelines: Expanded guidance about gynecologic cancers in BRCA1/2 carriers: Some highlights related to HRT include: Genetic/Familial High-Risk Assessment: Colorectal Cancer – Released …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2024-national-comprehensive-cancer-network-nccn-guideline-updates/
Permanent link to this article: https://inheritedcancer.net/post10524/
ICARE Newsletter Fall 2023
National Comprehensive Cancer Network (NCCN) Guidelines Updates
ICARE Newsletter Fall 2023
National Comprehensive Cancer Network (NCCN) Guidelines Updates
Check out the full NCCN guidelines by creating a FREE account at www.nccn.org Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic CancerReleased August 28th, 2023 (V1.2024) › Transgender, Non-Binary, and Gender Diverse Individuals: NEW section on care (Page 63-66, TNBGD-1 to 4)› Li-Fraumeni Syndrome: Significant updates to content (risks and care) (Pages 57-60, LIFR-A): Table added …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2023-national-comprehensive-cancer-network-nccn-guidelines-updates/
Permanent link to this article: https://inheritedcancer.net/post60823/
ICARE Newsletter Fall 2022
Ask the Expert
ICARE Newsletter Fall 2022
Ask the Expert
The below question was addressed by Dr. Kamran Idrees, Chief of the Division of Surgical Oncology & Endocrine Surgery, Associate Professor of Surgery, Ingram Associate Professor of Cancer Research, and Director of Pancreatic and Gastro Intestinal Surgical Oncology at Vanderbilt-Ingram Cancer Center. Dr. Idrees’ research has focused on colorectal cancer, liver metastases, and pancreatic cancer …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2022-ask-the-expert/
Newsletter Fall 2021
Updates to NCCN Genetic/Familial High-Risk Assessment
Newsletter Fall 2021
Updates to NCCN Genetic/Familial High-Risk Assessment
Breast, Ovarian, and Pancreatic Guidelines V.1.2022: Released August 11th, 2021 Colorectal Cancer Guidelines V.1.2021: Released May 11th, 2021 Check out the full NCCN guidelines by creating a FREE account at www.nccn.org
Permanent link to this article: https://inheritedcancer.net/newsletter-fall-2021-updates-to-nccn-genetic-familial-high-risk-assessment/
ICARE Social Media Post July 2022
Pancreatic Cancer Screening
ICARE Social Media Post July 2022
Pancreatic Cancer Screening
A recent study found that earlier diagnosis improved survival in people at high risk of pancreatic cancer.High risk was defined based on:family history and/orinherited gene mutation (BRCA1, BRCA2, CDKN2A, Lynch Syndrome genes, PALB2, ATM, and STK11)Read the article at the link: https://ascopost.com/news/june-2022/outcomes-of-pancreas-surveillance-in-the-caps5-study-and-total-caps-cohort/Reference: Dbouk, et al. J Clin Oncol. 2022 Jun 15:JCO2200298. doi: 10.1200/JCO.22.00298. PMID: 35704792.
Permanent link to this article: https://inheritedcancer.net/post72622/
ICARE Social Media Post March 2021
STK11: Cancer Risks and Risk Management
ICARE Social Media Post March 2021
STK11: Cancer Risks and Risk Management
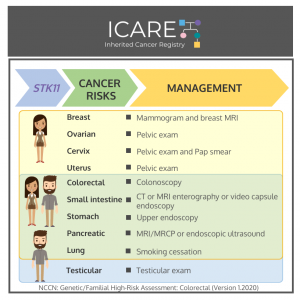
Gene: STK11 Cancer Risks and Management per National Comprehensive Cancer Network (NCCN) Genetic/Familial High-Risk Assessment: Colorectal Version 1.2020 Women:Breast cancer risk: Elevated at 40-60% – Recommend annual mammogram and breast MRI starting at around age 30. Ovarian tumor risk (typically benign sex cord/Sertoli cell tumors): Elevated at 18-21% – Recommend annual pelvic exam starting at …
Permanent link to this article: https://inheritedcancer.net/post33021/
ICARE Newsletter Winter 2020
Updates to National Comprehensive Cancer Network (NCCN) Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic
ICARE Newsletter Winter 2020
Updates to National Comprehensive Cancer Network (NCCN) Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic
There were significant updates and restructuring of the guidelines, with some highlights included below: Substantial reorganization of the guidelines as follows: Now organized by organ site, rather than primarily by certain high penetrance genes Focused efforts to simplify genetic testing criteria Only one flow diagram included, to outline the ‘genetic testing process’ Following scenarios now …
Permanent link to this article: https://inheritedcancer.net/1nlw2020/
ICARE Newsletter Winter 2020
Updated Pancreatic Cancer Screening Guidelines through CAPS Consortium
ICARE Newsletter Winter 2020
Updated Pancreatic Cancer Screening Guidelines through CAPS Consortium
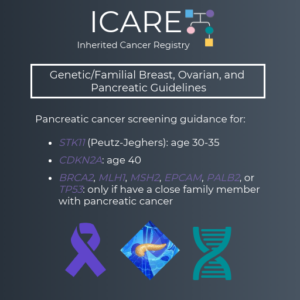
The International Cancer of the Pancreas Screening (CAPS) Consortium recently published updated recommendations about pancreatic cancer screening through MRI/magnetic retrograde cholangiopancreatography (MRCP) and/or an endoscopic ultrasound (EUS).1 Specifically, these guidelines now recommend that individuals with a CDKN2A or STK11 mutation begin screening at age 40. Screening for individuals with a BRCA1/2, ATM, PALB2, MLH1, or …
Permanent link to this article: https://inheritedcancer.net/4nlw2020/
ICARE Social Media Post February 2020
Differences in Pancreatic Cancer Screening Recommendations from the National Comprehensive Cancer Network (NCCN) and the International Cancer of the Pancreas Screening (CAPS) Consortium
ICARE Social Media Post February 2020
Differences in Pancreatic Cancer Screening Recommendations from the National Comprehensive Cancer Network (NCCN) and the International Cancer of the Pancreas Screening (CAPS) Consortium
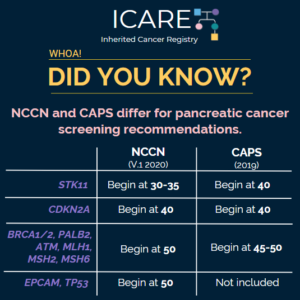
The National Comprehensive Cancer Network (NCCN) and the International Cancer of the Pancreas Screening (CAPS) Consortium recently updated pancreatic cancer screening recommendations. However, there are some differences between these recommendations. Specifically, screening with annual MRI/magnetic retrograde cholangiopancreatography (MRCP) and/or endoscopic ultrasound (EUS) is recommended as follows for NCCN versus CAPS: STK11 regardless of family history: …
Permanent link to this article: https://inheritedcancer.net/post2620/
ICARE Social Media Post February 2020
Updated Pancreatic Cancer Screening Guidelines through the International Cancer of the Pancreas Screening (CAPS) Consortium
ICARE Social Media Post February 2020
Updated Pancreatic Cancer Screening Guidelines through the International Cancer of the Pancreas Screening (CAPS) Consortium
The International Cancer of the Pancreas Screening (CAPS) Consortium recently published updated pancreatic cancer screening recommendations. The recommendations include: Screening with MRI/magnetic retrograde cholangiopancreaography (MRCP) and/or endoscopic ultrasound (EUS) The screening was recommended for the following individuals: CDKN2A and STK11 mutation carriers starting at age 40 BRCA1/2, ATM, PALB2, MLH1, and MSH2 mutation carriers (if …
Permanent link to this article: https://inheritedcancer.net/post2420/
ICARE Social Media Post December 2019
Updates to National Comprehensive Cancer Network (NCCN) Genetic/Familial Breast, Ovarian, and Pancreatic Guidelines (V1.2020)
ICARE Social Media Post December 2019
Updates to National Comprehensive Cancer Network (NCCN) Genetic/Familial Breast, Ovarian, and Pancreatic Guidelines (V1.2020)
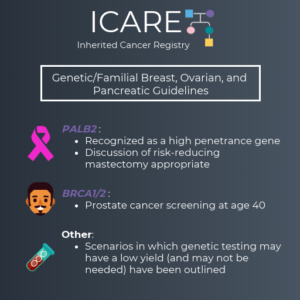
We are excited to share the latest version of the NCCN Genetic/Familial Breast, Ovarian and Pancreatic Guidelines (V1.2020), which were just updated. Some of the changes made include: PALB2 was added as a high penetrance gene (similar to BRCA1, BRCA2, CDH1, PTEN and TP53) It is appropriate to consider risk reducing mastectomy for cancer risk management …
Permanent link to this article: https://inheritedcancer.net/post12419/
Permanent link to this article: https://inheritedcancer.net/2nls2017/
Permanent link to this article: https://inheritedcancer.net/1nls2015/