Below is a featured video from the July 2025 case conference, during which Elizabeth Swisher, MD from the University of Washington discusses Myelodysplastic Syndrome (MDS) and Acute Myeloid Leukemia (AML) following PARP inhibitor therapy.
Care Consideration: PARP Inhibitors
Permanent link to this article: https://inheritedcancer.net/video71025/
ICARE Social Media Post May 2025
Inherited Prostate Cancer: PARP Inhibitors
ICARE Social Media Post May 2025
Inherited Prostate Cancer: PARP Inhibitors
A new meta-analysis study looking at prior studies of PARP inhibitors in patients with metastatic castration-resistant prostate cancer and an inherited gene mutation showed: Learn more at: https://pubmed.ncbi.nlm.nih.gov/39848867/ Reference: Naqvi, et al. Eur Urol. 2025:S0302-2838(24)02760-X. PMID: 39848867.
Permanent link to this article: https://inheritedcancer.net/post52125/
ICARE Newsletter Spring 2025
PARP Inhibitor: Relapsed BRCA-Mutated Ovarian Cancer
ICARE Newsletter Spring 2025
PARP Inhibitor: Relapsed BRCA-Mutated Ovarian Cancer
A new study (phase 3 ARIEL4 trial) to evaluate rucaparib (PARP inhibitor) versus standard-of-care chemotherapy among patients with relapsed BRCA-mutated ovarian cancer showed that median overall survival in the rucaparib group was 19.4 months versus 25.4 months in the chemotherapy group. This shows that more research is needed to figure out the most appropriate treatment …
Permanent link to this article: https://inheritedcancer.net/11nls2025/
Mar 24
ICARE Social Media Post March 2025
PARP-Inhibitor: Relapsed BRCA-Mutated Ovarian Cancer
ICARE Social Media Post March 2025
PARP-Inhibitor: Relapsed BRCA-Mutated Ovarian Cancer
A new study (phase 3 ARIEL4 trial) to evaluate rucaparib (PARP Inhibitor) versus standard-of-care chemotherapy among patients with relapsed BRCA-mutated ovarian cancer showed median overall survival in the rucaparib group was 19.4 months versus 25.4 months in the chemotherapy group. This shows that more research is needed to figure out the most appropriate treatment options …
Permanent link to this article: https://inheritedcancer.net/post32425/
ICARE Social Media Post January 2025
1 Year of Olaparib Improves Outcomes in BRCA Breast Cancer Patients
ICARE Social Media Post January 2025
1 Year of Olaparib Improves Outcomes in BRCA Breast Cancer Patients
A new study presented at the 2024 San Antonio Breast Cancer Symposium suggests:⤷ Giving PARP Inhibitor (Olaparib) for one year after standard cancer treatment led to benefits in BRCA-mutation breast cancers. What was seen? These findings bring up the possibility of considering using PARP inhibitors in BRCA carriers: To learn more, read the full article …
Permanent link to this article: https://inheritedcancer.net/post10925/
ICARE Social Media Post November 2024
BRCA1/2 Carriers with Advanced Breast Cancer
ICARE Social Media Post November 2024
BRCA1/2 Carriers with Advanced Breast Cancer
As highlighted in our latest ICARE newsletter, a new study in BRCA1/2 carriers with advanced breast cancer found: Read the full article to learn more at: https://pubmed.ncbi.nlm.nih.gov/37437366/Reference: Valenza, et al. Eur J Cancer. 2023;190:112944. PMID: 37437366. We also encourage you to read the full ICARE newsletter for other clinical and research updates at https://inheritedcancer.net/newsletters/
Permanent link to this article: https://inheritedcancer.net/icare-social-media-post-november-2024-brca1-2-carriers-with-advanced-breast-cancer/
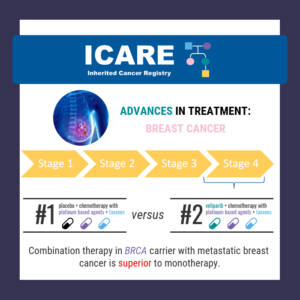
ICARE Social Media Post November 2024
BRCA Carriers with Breast Cancer: Trial to Compare PARP Inhibitors to Chemotherapy
ICARE Social Media Post November 2024
BRCA Carriers with Breast Cancer: Trial to Compare PARP Inhibitors to Chemotherapy
As highlighted in the latest ICARE newsletter, a recent trial among BRCA1/2 carriers with breast cancer comparing PARP inhibitors to standard chemotherapy (Treatment of Physician’s Choice – TPC) found that after 25.7 months of follow up: Overall survival in each group was: The % alive at 3 years was: Patients who received Olaparib for first …
Permanent link to this article: https://inheritedcancer.net/post111224/
ICARE Newsletter Fall 2024
BRCA1/2 Carriers: Treatment Advances
ICARE Newsletter Fall 2024
BRCA1/2 Carriers: Treatment Advances
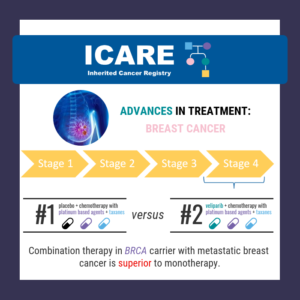
Among BRCA1/2 carriers with advanced breast cancer, PARP inhibitors showed some activity even in patients with platinum resistant/unresponsive disease. However, the optimal delivery of platinum agents and PARP inhibitors was not clear.1 In another study of BRCA1/2 carriers with breast cancer (OlympiAD Trial), PARP inhibitors were compared to chemotherapy Treatment of Physician’s Choice (TPC), with …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2024-brca1-2-carriers-treatment-advances/
ICARE Newsletter Spring 2024
BRCA-Associated Prostate Cancer Treatment Updates
ICARE Newsletter Spring 2024
BRCA-Associated Prostate Cancer Treatment Updates
New studies to guide treatment strategies in men with prostate cancer and a BRCA mutation were recently published. Specifically, a recent study suggested that platinum-based chemotherapy may be as effective as PARP inhibitors for individuals with BRCA-positive metastatic castration-resistant prostate cancer.1 The study sheds light on treatment options for advanced prostate cancer patients. More recently, …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2024-brca-associated-prostate-cancer-treatment-updates/
PARP Inhibitor (Olaparib) in men with BRCA mutations and prostate cancer
A recent study found that Olaparib (Lynparza) improved survival outcomes among men with BRCA1/2 mutations and metastatic castration-resistant prostate cancer, regardless of whether the mutation was germline or somatic. This underscores the potential of targeted therapies in improving outcomes for those with inherited cancer gene mutations. Learn more at: https://ascopubs.org/doi/10.1200/JCO.23.00339 Reference: Mateo, et al. J …
Permanent link to this article: https://inheritedcancer.net/post22024/
ICARE Social Media Post January 2023
BRCA/Prostate Cancer/Treatment
ICARE Social Media Post January 2023
BRCA/Prostate Cancer/Treatment
A recent study suggests that Platinum Chemotherapy is as effective as PARP inhibitors for individuals with BRCA-positive metastatic castration-resistant prostate cancer. The study sheds light on treatment options for advanced prostate cancer patients 🩺✨ Learn more at: http://tinyurl.com/3vs2mk8f Reference: Fazekas, et al. Eur Urol Oncol. 2023: S2588-9311(23)00174-8. PMID: 37722977.
Permanent link to this article: https://inheritedcancer.net/post10324/
ICARE Newsletter Fall 2023
BRCA-associated Prostate Cancers
ICARE Newsletter Fall 2023
BRCA-associated Prostate Cancers
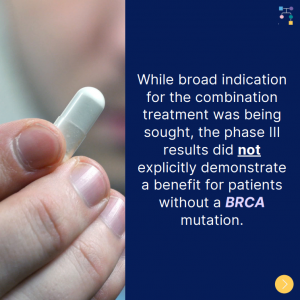
On April 28th, 2023, the FDA approved olaparib plus abiraterone acetate for first line treatment for metastatic castration-resistant prostate cancer, but only in patients whose tumors have BRCA mutations. Although a broad indication for the combination therapy was desired, concerns about the trial design were raised, and the phase III results did not explicitly show …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2023-brca-associated-prostate-cancers/
ICARE Social Media Post June 2023
FDA Advisory Committee Recommendation: Olaparib for Prostate Cancer Treatment
ICARE Social Media Post June 2023
FDA Advisory Committee Recommendation: Olaparib for Prostate Cancer Treatment
Olaparib plus abiraterone acetate is recommended as the first line treatment for metastatic castration-resistant prostate cancer, but only in patients whose tumors have BRCA mutations. Although a broad indication for the combination therapy was desired, concerns about the trial design were raised, and the phase III results did not explicitly show that patients without a …
Permanent link to this article: https://inheritedcancer.net/post61623/
ICARE Social Media Post May 2023
PARP Inhibitors for Metastatic Prostate Cancer: Talazoparib
ICARE Social Media Post May 2023
PARP Inhibitors for Metastatic Prostate Cancer: Talazoparib
A study that compared a PARP inhibitor (talazoparib) to the standard of care (an androgen receptor inhibitor) in metastatic castration-resistant prostate cancer found that it improved progression-free survival regardless of BRCA mutation status. However, the benefit was greatest in males with a BRCA or other DNA repair gene mutation. Read the full article at this …
Permanent link to this article: https://inheritedcancer.net/post50823/
ICARE Social Media Post April 2023
PARP Inhibitors for Metastatic Prostate Cancer: Niraparib
ICARE Social Media Post April 2023
PARP Inhibitors for Metastatic Prostate Cancer: Niraparib
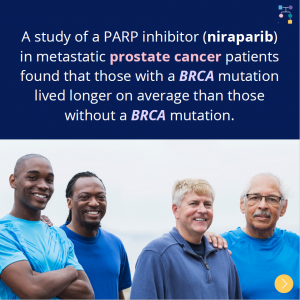
A study that evaluated a PARP inhibitor (niraparib) in males with metastatic prostate cancer found that those with a BRCA1 or BRCA2 mutation lived longer on average than those who did not have a BRCA mutation. Read the full article at the link: https://pubmed.ncbi.nlm.nih.gov/35131040/Reference:Smith et al. Lancet Oncol. 2022;23(3):362-373. PMID: 35131040.
Permanent link to this article: https://inheritedcancer.net/post42023/
ICARE Newsletter Spring 2023
Prostate Cancer Treatment Updates
ICARE Newsletter Spring 2023
Prostate Cancer Treatment Updates
A study to test niraparib (a PARP inhibitor) in males with metastatic prostate cancer showed that those with an inherited BRCA1 or BRCA2 (BRCA) mutation lived longer on average compared to those without a BRCA mutation. Side effects from niraparib were similar to those previously reported with PARP inhibitors.1 Another PARP inhibitor trial tested an …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2023prostate-cancer-treatment-updates/
ICARE Newsletter Spring 2023
Breast Cancer Treatment Updates
ICARE Newsletter Spring 2023
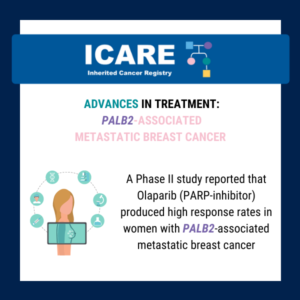
Findings from a Phase II study to evaluate the use of talazoparib (a PARP inhibitor) in individuals with advanced PALB2-mutation breast cancer showed that it appeared effective in certain patients and appeared safe (with similar adverse events as those previously reported with this drug).1 There are several Phase II trials to evaluate PARP inhibitors in …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2023-breast-cancer-treatment-updates/
ICARE Social Media Post March 2023
Treatment for PALB2-associated breast cancer with Talazoparib
ICARE Social Media Post March 2023
Treatment for PALB2-associated breast cancer with Talazoparib
According to a recent Phase II study, Talazoparib may benefit patients with PALB2-associated breast cancer. Findings showed that Talazoparib appeared effective and safe in certain patients with advanced disease. Read the full article at this link: https://www.nature.com/articles/s43018-022-00439-1 Reference: Gruber et al. Nat Cancer. 2022;3(10):1181-1191. PMID: 36253484.
Permanent link to this article: https://inheritedcancer.net/post32723/
ICARE Newsletter Spring 2022
Inherited Cancer Treatment Updates
ICARE Newsletter Spring 2022
Inherited Cancer Treatment Updates
Lynch Syndrome Carriers with Advanced Uterine Cancer: Treatment with PembrolizumabWomen with Lynch Syndrome are at high risk for uterine cancer. The type of uterine cancer they develop has the tumorcharacteristic of being ‘MSI-H’. A new study indicated treatment with pembrolizumab (Keytruda) resulted in benefit inpatients with MSI-H advanced uterine cancer. Von Hippel-Lindau Patients: Treatment of …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2022-inherited-cancer-treatment-updates/
ICARE Newsletter Fall 2022
Screening & Treatment Updates: Pancreatic Cancer
ICARE Newsletter Fall 2022
Screening & Treatment Updates: Pancreatic Cancer
A recent small study suggests that immunotherapy may benefit patients with refractory pancreatic or biliary cancer who have inherited a mutation in the homologous recombination deficiency (HRD) genes, BRCA1, BRCA2, and RAD51C.Another new study reported that in BRCA1/2 carriers with pancreatic cancer, maintenance treatment with Olaparib may be of benefit. Findings showed that with Olaparib, …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2022-screening-treatment-updates-pancreatic-cancer/
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2021-ask-the-expert/
Newsletter Fall 2021
Inherited Cancer Treatment Updates
Newsletter Fall 2021
Inherited Cancer Treatment Updates
Early-stage, high-risk breast cancer in BRCA carriers: Results of the highly awaited phase 3 OlympiA trial showed promising results for EARLY STAGE (i.e., localized Stage 2-3) high-risk breast cancer patients with a BRCA mutation who were treated with a PARP inhibitor (olaparib) in the adjuvant setting (i.e., AFTER surgery).1 Early-stage breast cancer in this trial …
Permanent link to this article: https://inheritedcancer.net/newsletter-fall-2021-inherited-cancer-treatment-updates/
Newsletter Fall 2021
Updates to NCCN Genetic/Familial High-Risk Assessment
Newsletter Fall 2021
Updates to NCCN Genetic/Familial High-Risk Assessment
Breast, Ovarian, and Pancreatic Guidelines V.1.2022: Released August 11th, 2021 Colorectal Cancer Guidelines V.1.2021: Released May 11th, 2021 Check out the full NCCN guidelines by creating a FREE account at www.nccn.org
Permanent link to this article: https://inheritedcancer.net/newsletter-fall-2021-updates-to-nccn-genetic-familial-high-risk-assessment/
ICARE Social Media Post August 2022
Pancreatic Cancer Treatment
ICARE Social Media Post August 2022
Pancreatic Cancer Treatment
A new study reports that maintenance treatment with Olaparib may benefit BRCA1/2 carriers with pancreatic cancer. These findings demonstrated:long-term survival was more commontime to subsequent therapy was prolongedRead the full article at the link: https://ascopubs.org/doi/pdf/10.1200/JCO.21.01604Reference: Kindler et al. J Clin Oncol. 2022; JCO2101604. PMID: 35834777.
Permanent link to this article: https://inheritedcancer.net/post80522/
ICARE Social Media Post May 2022
BRCA Carriers with Risk-Reducing Salpingo-oophorectomy: Risk of Peritoneal Carcinomatosis
ICARE Social Media Post May 2022
BRCA Carriers with Risk-Reducing Salpingo-oophorectomy: Risk of Peritoneal Carcinomatosis
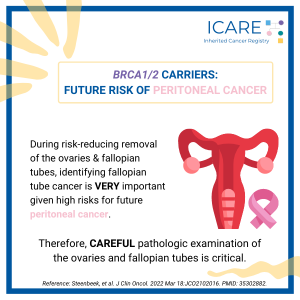
A new study found that among BRCA1/2 carriers, the presence of tubal intraepithelial carcinoma during risk-reducing salpingo-oophorectomy (i.e., preventive surgery to remove the ovaries and fallopian tubes) predicts the risk of later peritoneal cancer. These findings demonstrate:the importance of timely risk-reducing removal of the ovaries and fallopian tubesthat it is VERY important to have a …
Permanent link to this article: https://inheritedcancer.net/post52422/
ICARE Social Media Post April 2022
Disparities: PARP Inhibitors & Ovarian Cancer
ICARE Social Media Post April 2022
Disparities: PARP Inhibitors & Ovarian Cancer
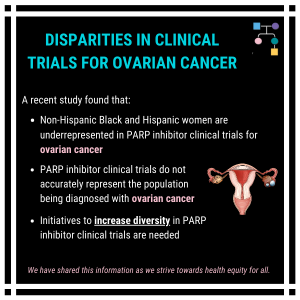
A recent study found that non-Hispanic Black and Hispanic women are underrepresented in clinical trials evaluating PARP inhibitor use among ovarian cancer patients; therefore, these trials do not accurately represent the ovarian cancer patient population. These findings indicate initiatives to increase diversity in clinical trials are needed. Read the full article for more information 👇https://www.gynecologiconcology-online.net/article/S0090-8258(22)00071-3/fulltext#secst0030Reference: …
Permanent link to this article: https://inheritedcancer.net/post42222/
ICARE Social Media Post April 2022
BRCA1/2 Carriers with Breast Cancer: Olaparib & Survival
ICARE Social Media Post April 2022
BRCA1/2 Carriers with Breast Cancer: Olaparib & Survival
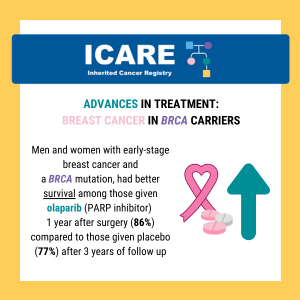
A new study found that adjuvant olaparib significantly extended survival in BRCA1/2 carriers with HER2-negative high-risk early-stage breast cancer. Learn more at the following link: https://www.healio.com/news/hematology-oncology/20220323/adjuvant-olaparib-prolongs-survival-for-certain-patients-with-early-breast-cancer
Permanent link to this article: https://inheritedcancer.net/post41922/
Permanent link to this article: https://inheritedcancer.net/post40822/
ICARE Social Media Post March 2022
FDA Approves Olaparib for Adjuvant Treatment of High-risk Early Breast Cancer
ICARE Social Media Post March 2022
FDA Approves Olaparib for Adjuvant Treatment of High-risk Early Breast Cancer
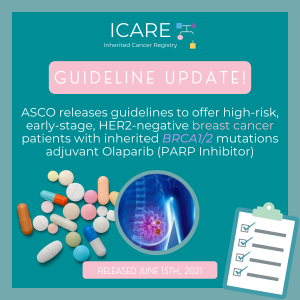
On March 11th, the Food and Drug Administration (FDA) approved olaparib (Lynparza) for the adjuvant treatment of BRCA1/2 carriers with human epidermal growth factor receptor 2 (HER2)-negative, high-risk early breast cancer who have been treated with neoadjuvant or adjuvant chemotherapy.Learn more at 👇https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-olaparib-adjuvant-treatment-high-risk-early-breast-cancer
Permanent link to this article: https://inheritedcancer.net/post31522/
Permanent link to this article: https://inheritedcancer.net/post10422/
ICARE Social Media Post December 2021
Fall 2021 Ask the Expert
ICARE Social Media Post December 2021
Fall 2021 Ask the Expert
In every ICARE newsletter we give our participants the opportunity to have a question addressed by an expert in the field. In the latest edition, Dr. Kerry Schaffer discusses the use of PARP inhibitors to treat inherited forms of prostate cancer.Check out Dr. Schaffer’s full response at 👇https://inheritedcancer.net/newsletters/
Permanent link to this article: https://inheritedcancer.net/post122821/
ICARE Social Media Post December 2021
FDA Approves PARP Inhibitor (Olaparib) Treatment for some BRCA carriers with Early Stage Breast Cancer
ICARE Social Media Post December 2021
FDA Approves PARP Inhibitor (Olaparib) Treatment for some BRCA carriers with Early Stage Breast Cancer
FDA granted priority review to Olaparib for adjuvant treatment of certain patients with high-risk breast cancer. This designation applies to use of the agent by patients with BRCA-mutated, HER2-negative, high-risk early breast cancer who receive chemotherapy before or after surgery. For additional information, visit: https://tinyurl.com/healioFDAapproval
Permanent link to this article: https://inheritedcancer.net/post120621/
ICARE Social Media Post August 2021
ASCO Guideline Update: Olaparib for Breast Cancer
ICARE Social Media Post August 2021
ASCO Guideline Update: Olaparib for Breast Cancer
For additional information, read the updated American Society of Clinical Oncology (ASCO) recommendation (released June 15th, 2021) at the following link: https://www.asco.org/practice-patients/guidelines/breast-cancer?intcmp=ws_ascoorg_gdlns_hereditarybreastcancer_site_pressrelease_061621____#/143725
Permanent link to this article: https://inheritedcancer.net/post81021/
Permanent link to this article: https://inheritedcancer.net/post71621/
Permanent link to this article: https://inheritedcancer.net/post70921/
ICARE Social Media Post July 2021
Advances in Pancreatic Cancer Treatment: PALB2 & BRCA1/2
ICARE Social Media Post July 2021
Advances in Pancreatic Cancer Treatment: PALB2 & BRCA1/2
For more information, view the article at the following link below: https://ascopubs.org/doi/abs/10.1200/JCO.21.00003 You may also read the ASCO post article at: https://ascopost.com/news/may-2021/maintenance-rucaparib-in-patients-with-platinum-sensitive-pancreatic-cancer-and-germline-or-somatic-brca1-brca2-or-palb2-variants/?utm_source=TAP%2DEN%2D051221%2DTrending%5FLymphoma&utm_medium=email&utm_term=49cf1c97d48c2cf8231827e3bcb15769
Permanent link to this article: https://inheritedcancer.net/post70621/
Permanent link to this article: https://inheritedcancer.net/post61121/
Permanent link to this article: https://inheritedcancer.net/post41321/
Permanent link to this article: https://inheritedcancer.net/post22321/
ICARE Newsletter Winter 2021
Inherited Cancer Treatment: Updates and Relevant Policies
ICARE Newsletter Winter 2021
Inherited Cancer Treatment: Updates and Relevant Policies
Over the last several months, the American Society of Clinical Oncology published a number of guidelines related to the use of PARP inhibitors among those with BRCA-associated cancers, including guidelines focused on ovarian cancer,1 metastatic pancreatic cancer,2 and breast cancer.3 Additionally, costs of drugs also have great potential to influence policy, highlighting the importance of …
Permanent link to this article: https://inheritedcancer.net/7nlw2021/
Permanent link to this article: https://inheritedcancer.net/post122220/
Permanent link to this article: https://inheritedcancer.net/post121120/
Permanent link to this article: https://inheritedcancer.net/post112720/
ICARE Social Media Post November 2020
ASCO Guideline Updates: Breast Cancer
ICARE Social Media Post November 2020
ASCO Guideline Updates: Breast Cancer
The American Society of Clinical Oncology (ASCO) published updated guidelines for the management of hereditary breast cancer for the following gene carriers: 𝘽𝙍𝘾𝘼1/2 • Consider breast-conserving therapy • Consider nipple-sparing mastectomy, if medically appropriate • Advanced breast cancer: ⫸ PARP inhibitors (olaparib, talazoparib) preferred over non-platinum single agent chemotherapy ⫸ Platinum agents are recommended …
Permanent link to this article: https://inheritedcancer.net/post112020/
ICARE Social Media Post November 2020
Clinical Trial Participation Powers Patient’s Positive Attitude
ICARE Social Media Post November 2020
Clinical Trial Participation Powers Patient’s Positive Attitude
Brooke Thomas has leaned on 12 years of experience as a medical social worker and found ways to stay positive and upbeat through it all – and she has a lot to be positive about these days, thanks to an amazing response to her treatment as part of a clinical trial at Vanderbilt-Ingram Cancer Center. …
Permanent link to this article: https://inheritedcancer.net/post110620/
ICARE Social Media Post October 2020
New ASCO Guidelines On Use Of PARP Inhibitors To Manage Ovarian Cancer
ICARE Social Media Post October 2020
New ASCO Guidelines On Use Of PARP Inhibitors To Manage Ovarian Cancer
New guidelines for the use of PARP inhibitors to treat ovarian cancer among those with BRCA1 or BRCA2 mutations were published through the American Society of Clinical Oncology (ASCO) to guide providers about the role of this class of drugs in the management of this type of cancer. Link to the guidelines are available at: …
Permanent link to this article: https://inheritedcancer.net/post101320/
ICARE Social Media Post October 2020
Addition of Veliparib to Carboplatin/Paclitaxel in Previously Treated Patients With BRCA-Mutated Advanced Breast Cancer
ICARE Social Media Post October 2020
Addition of Veliparib to Carboplatin/Paclitaxel in Previously Treated Patients With BRCA-Mutated Advanced Breast Cancer
Among BRCA carriers with metastatic breast cancer, the combination of veliparib AND chemotherapy with platinum-based agents (carboplatin) and taxanes (paclitaxel) led to a longer duration of progression free survival (disease that did not progress), compared to those treated with ONLY chemotherapy. To read the full article visit: https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(20)30447-2/fulltext [This finding was previously outlined last year …
Permanent link to this article: https://inheritedcancer.net/post100620/
Permanent link to this article: https://inheritedcancer.net/post91820/
ICARE Social Media Post May 2020
Advances in Treatment for Metastatic Prostate Cancer: Olaparib
ICARE Social Media Post May 2020
Advances in Treatment for Metastatic Prostate Cancer: Olaparib
On May 19, 2020 the FDA approved the use of olaparib (Lynparza) as treatment in BRCA and other gene carriers (homologous recombination repair genes) with metastatic castration-resistant prostate cancer who have been treated with enzalutamide or abiraterone. Link to full article: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-olaparib-hrr-gene-mutated-metastatic-castration-resistant-prostate-cancer
Permanent link to this article: https://inheritedcancer.net/post52220/
ICARE Social Media Post May 2020
Advances in Treatment for Metastatic Prostate Cancer: Rucaparib
ICARE Social Media Post May 2020
Advances in Treatment for Metastatic Prostate Cancer: Rucaparib
On May 15, 2020 the FDA approved the use of rucaparib (Rubraca) as treatment in BRCA carriers with metastatic castration-resistant prostate cancer who have been treated with androgen receptor-directed therapy and a taxane-based chemotherapy. Link to full article: https://www.fda.gov/drugs/fda-grants-accelerated-approval-rucaparib-brca-mutated-metastatic-castration-resistant-prostate
Permanent link to this article: https://inheritedcancer.net/post51920/
ICARE Social Media Post May 2020
Advances in Ovarian Cancer Treatment for BRCA1/2 Carriers: Olaparib & bevacizumab
ICARE Social Media Post May 2020
Advances in Ovarian Cancer Treatment for BRCA1/2 Carriers: Olaparib & bevacizumab
On May 8, 2020 the FDA approved the use of olaparib (Lynparza) as first-line maintenance treatment in BRCA1/2 carriers (deleterious or suspected deleterious mutations) and/or a genomic instability, with advanced epithelial ovarian, fallopian tube or primary peritoneal cancer who are in complete or partial response to first-line platinum-based chemotherapy. Link to full article: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-olaparib-plus-bevacizumab-maintenance-treatment-ovarian-fallopian-tube-or-primary
Permanent link to this article: https://inheritedcancer.net/post51220/
ICARE Social Media Post May 2020
Advances in Ovarian Cancer Treatment for BRCA1/2 Carriers: Olaparib
ICARE Social Media Post May 2020
Advances in Ovarian Cancer Treatment for BRCA1/2 Carriers: Olaparib
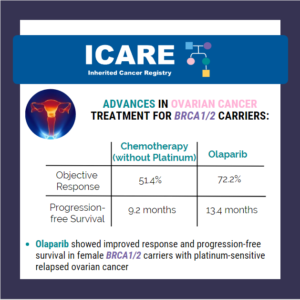
In recognition of World Ovarian Cancer Day, we’d like to share some exciting results from a study of women with ovarian cancer and a BRCA mutation: In a recent phase III trial, olaparib (PARP inhibitor) showed improved response and progression-free survival compared with chemotherapy (without platinum) in BRCA carriers with platinum-sensitive relapsed ovarian cancer who …
Permanent link to this article: https://inheritedcancer.net/post50820/
ICARE Social Media Post May 2020
Advances in Treatment for Metastatic Prostate Cancer: Olaparib
ICARE Social Media Post May 2020
Advances in Treatment for Metastatic Prostate Cancer: Olaparib
Findings from a recent study showed that olaparib (PARP inhibitor) significantly improved progression-free survival in patients with BRCA1, BRCA2, or ATM genetic alterations. Benefits were also more broadly seen among patients with homologous recombination repair gene defects. Link to full article: https://www.nejm.org/doi/full/10.1056/NEJMoa1911440 Check out the ASCO post article at: https://www.ascopost.com/news/may-2020/olaparib-for-patients-with-mcrpc-and-homologous-recombination-repair-gene-alterations/
Permanent link to this article: https://inheritedcancer.net/post50620/
ICARE Social Media Post April 2020
Advances in Treatment for Ovarian Cancer in BRCA1/2 Carriers: Niraparib
ICARE Social Media Post April 2020
Advances in Treatment for Ovarian Cancer in BRCA1/2 Carriers: Niraparib
On April 29, 2020 the FDA approved the use of niraparib (Zeluja) as first-line maintenance treatment in BRCA1/2 carriers with advanced ovarian cancer! More details available at: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-niraparib-first-line-maintenance-advanced-ovarian-cancer
Permanent link to this article: https://inheritedcancer.net/post42920/
ICARE Newsletter Winter 2020
Treatment Advances Among Those with Inherited Prostate Cancer Predisposition
ICARE Newsletter Winter 2020
Treatment Advances Among Those with Inherited Prostate Cancer Predisposition
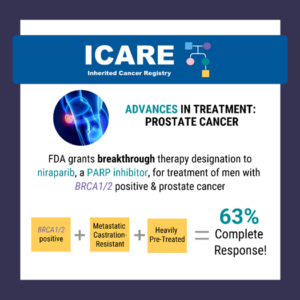
A recent study reported a high complete response rate among men with a BRCA1/2 mutation with metastatic, castration-resistant prostate cancer who were treated with niraparib (a PARP inhibitor) of 63% compared to 17% in the non-BRCA1/2 group.1 Based on this data, the Federal Drug Administration (FDA) granted breakthrough therapy designation to niraparib on October 3, …
Permanent link to this article: https://inheritedcancer.net/3nlw2020/
ICARE Newsletter Winter 2020
Treatment Advances Among Those with Inherited Pancreatic Cancer Predisposition
ICARE Newsletter Winter 2020
Treatment Advances Among Those with Inherited Pancreatic Cancer Predisposition
Results from a recent study showed olaparib (a PARP inhibitor) nearly doubled the progression-free survival in BRCA1/2 carriers with metastatic pancreatic cancer.1 Based on this data, the FDA approved the use of olaparib as a first-line maintenance treatment in BRCA1/2 carriers with metastatic, platinum-sensitive pancreatic cancer. This represents another treatment advance in pancreatic cancer and …
Permanent link to this article: https://inheritedcancer.net/2nlw2020/
ICARE Social Media Post January 2020
Celebrating 10 Years of ICARE
ICARE Social Media Post January 2020
Celebrating 10 Years of ICARE
Happy New Year! 2020 represents a decade for ICARE We are celebrating 10 years of research, education, and engagement, through which we have enrolled nearly 3500 participants, including over 2000 gene mutation carriers, disseminated 15 newsletters, led and collaborated on multiple research projects, and impacted individuals affected by inherited cancer predisposition all over the …
Permanent link to this article: https://inheritedcancer.net/post1920/
ICARE Social Media Post January 2020
Advances in Treatment for Pancreatic Cancer in BRCA Carriers
ICARE Social Media Post January 2020
Advances in Treatment for Pancreatic Cancer in BRCA Carriers
The FDA approved the use of olaparib, a PARP inhibitor, as first-line maintenance treatment in BRCA1/2 carriers with metastatic, platinum-sensitive, pancreatic cancer. Platinum-sensitive cancer is a cancer that responds to treatment with drugs that contain the metal platinum, such as carboplatin or cisplatin. Olaparib showed to nearly double the progression-free survival in BRCA1/2 carriers with …
Permanent link to this article: https://inheritedcancer.net/post1320/
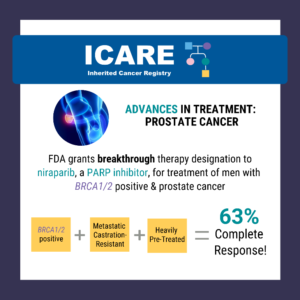
ICARE Social Media Post December 2019
Evaluation of PARP Inhibitors in BRCA-Associated Prostate Cancer
ICARE Social Media Post December 2019
Evaluation of PARP Inhibitors in BRCA-Associated Prostate Cancer
The FDA granted breakthrough therapy designation to niraparib (a PARP inhibitor) for the treatment of men with BRCA1/2 positive, metastatic castration-resistant, and heavily pre-treated prostate cancer. Results from a recent study show a 63% complete response rate in men with BRCA1/2 positive, metastatic castration-resistant prostate cancer treated with niraparib compared to 17% in the non- …
Permanent link to this article: https://inheritedcancer.net/post122019/
ICARE Social Media Post December 2019
Patient Reported Outcomes In A Study of PARP Inhibitors in BRCA Carriers with Metastatic Breast Cancer
ICARE Social Media Post December 2019
Patient Reported Outcomes In A Study of PARP Inhibitors in BRCA Carriers with Metastatic Breast Cancer
Did you know? PROs are impacting treatment advances in metastatic breast cancer. Olaparib increased progression-free survival among BRCA carriers with metastatic HER2- breast cancer. Thanks to patient reported outcomes, a new study now suggests it also improved patients’ quality of life! Check it out the new article published in October 2019 directly at https://www.ncbi.nlm.nih.gov/pubmed/31446213!
Permanent link to this article: https://inheritedcancer.net/post12819/
ICARE Social Media Post November 2019
High Frequency of BRCA in Unselected Women with Metastatic Breast Cancer
ICARE Social Media Post November 2019
High Frequency of BRCA in Unselected Women with Metastatic Breast Cancer
Did you know? National practice guidelines currently recommend ALL women with metastatic (HER2-) breast cancer to get genetic testing for inherited cancer (including BRCA1/2 testing), because it can guide eligibility for treatment with PARP inhibitors. A new study led by our colleague at the Vanderbilt-Ingram Cancer Center, Dr. Ben Park, suggests that more women with …
Permanent link to this article: https://inheritedcancer.net/post110719/
ICARE Social Media Post October 2019
Advances in Treatment for Advanced Breast Cancer in BRCA Carriers
ICARE Social Media Post October 2019
Advances in Treatment for Advanced Breast Cancer in BRCA Carriers
Monotherapy with PARP inhibitors is FDA-approved for patients with metastatic breast cancer with BRCA mutations. BUT, does adding additional drugs (called ‘combination therapy’) help? In BRCA carriers with metastatic breast cancer, the combination of veliparib AND chemotherapy with platinum-based agents (carboplatin) and taxanes (paclitaxel) led to a longer duration of progression free survival (disease that …
Permanent link to this article: https://inheritedcancer.net/post101819/
ICARE Social Media Post October 2019
Advances in Early Stage Breast Cancer Treatment for BRCA Carriers
ICARE Social Media Post October 2019
Advances in Early Stage Breast Cancer Treatment for BRCA Carriers
New benefits from a PARP inhibitor, talazoparib, among BRCA carriers with early stage breast cancer. The current FDA approvals for the use of PARP inhibitors is limited to women with metastatic (stage IV) breast cancer. These drugs are being tested in early stage breast cancer to shrink down the tumor (called neoadjuvant treatment) before surgery …
Permanent link to this article: https://inheritedcancer.net/post101519/
ICARE Newsletter Summer 2019
Ovarian Cancer Treatment Advances for BRCA1/2 Carriers
ICARE Newsletter Summer 2019
Ovarian Cancer Treatment Advances for BRCA1/2 Carriers
A recently reported study of women with ovarian cancer and homologous recombination deficiency (HRD) who received a PARP inhibitor (niraparib) as fourth line or later treatment showed potential clinical benefit. Specifically, median overall survival after treatment was 19 months in the HRD-positive group (including those with BRCA1/2 mutations) compared to 15.5 months in the HRD-negative …
Permanent link to this article: https://inheritedcancer.net/4nls2019/
ICARE Newsletter Summer 2019
Pancreatic Cancer Treatment Advances for BRCA1/2 Carriers
ICARE Newsletter Summer 2019
Pancreatic Cancer Treatment Advances for BRCA1/2 Carriers
Results from a clinical trial of individuals with a BRCA1/2 mutation and pancreatic cancer showed that patients who received a PARP inhibitor (olaparib) for maintenance treatment had almost half the risk of their disease progressing when compared to receiving a placebo.1 In fact, after 2 years, 22.1% of patients who received olaparib had no disease …
Permanent link to this article: https://inheritedcancer.net/3nls2019/
ICARE Newsletter Summer 2019
Prostate Cancer Treatment Advances for BRCA1/2 Carriers
ICARE Newsletter Summer 2019
Prostate Cancer Treatment Advances for BRCA1/2 Carriers
There is now information to suggest that identifying inherited mutations in DNA repair genes, such as BRCA1/2 and other genes, in men with metastatic prostate cancer may open doors for other treatment options. Results of a phase 2 clinical trial among men with metastatic and heavily pre-treated prostate cancer were presented at the American Society …
Permanent link to this article: https://inheritedcancer.net/2nls2019/
ICARE Newsletter Winter 2019
New Research and Approvals of PARP Inhibitor Drugs to Treat Prostate Cancer in BRCA Carriers
ICARE Newsletter Winter 2019
New Research and Approvals of PARP Inhibitor Drugs to Treat Prostate Cancer in BRCA Carriers
Treatment among patients with metastatic castration-resistant prostate cancer: A PARP inhibitor (rucaparib) was granted a breakthrough therapy designation in October 2018 for monotherapy (i.e., sole treatment) among men with metastatic castration-resistant prostate cancer (with a BRCA1/2 mutation) who have received at least one prior androgen receptor-directed treatment and taxane-based chemotherapy. This designation was granted based …
Permanent link to this article: https://inheritedcancer.net/3nlw2019/
ICARE Newsletter Winter 2019
New Research and Approvals of PARP Inhibitor Drugs to Treat Breast Cancer in BRCA Carriers
ICARE Newsletter Winter 2019
New Research and Approvals of PARP Inhibitor Drugs to Treat Breast Cancer in BRCA Carriers
Treatment among patients with advanced or metastatic breast cancer: A PARP inhibitor (talazoparib) was approved by the FDA on October 16, 2018 for BRCA carriers with HER2-negative locally advanced or metastatic breast cancer, based on results of the EMBRACA trial outlined in the last ICARE newsletter. Litton JK, et al. N Engl J Med. 2018 …
Permanent link to this article: https://inheritedcancer.net/1nlw2019/
ICARE Newsletter Winter 2019
New Research and Approvals of PARP Inhibitor Drugs to Treat Ovarian Cancer in BRCA Carriers
ICARE Newsletter Winter 2019
New Research and Approvals of PARP Inhibitor Drugs to Treat Ovarian Cancer in BRCA Carriers
First line maintenance treatment among patients newly diagnosed with advanced ovarian cancer: The results of a trial using a PARP inhibitor (olaparib) as maintenance treatment among ovarian cancer patients with advanced disease, a BRCA mutation, and complete or partial response to platinum-based chemotherapy showed that survival at 3 years was 60% among those who got …
Permanent link to this article: https://inheritedcancer.net/2nlw2019/
ICARE Newsletter Summer 2018
Another PARP-Inhibitor Trial Among BRCA Carriers with Advanced Breast Cancer
ICARE Newsletter Summer 2018
Another PARP-Inhibitor Trial Among BRCA Carriers with Advanced Breast Cancer
In a Phase 3 clinical trial among BRCA carriers with advanced breast cancer, an oral PARP Inhibitor (talazoparib) was compared to standard chemotherapy. Among those who received the PARP inhibitor, risk of disease progression or death was 46% lower, and the response rate was double. Furthermore, the side effect profile, quality-of-life measures, and breast cancer …
Permanent link to this article: https://inheritedcancer.net/8nls2018/
ICARE Newsletter Winter 2018
The Role of “Non-Truncating” Mutations in RAD51D on Ovarian Cancer Risk
ICARE Newsletter Winter 2018
The Role of “Non-Truncating” Mutations in RAD51D on Ovarian Cancer Risk
As testing has broadened to include newer inherited cancer genes, studies have suggested that mutations which shorten the protein (“truncating mutations”) in the RAD51D gene are associated with ovarian cancer. However, a recent study examined ovarian cancer risks for a “non-truncating” change (a single base pair within the gene is changed which is called a …
Permanent link to this article: https://inheritedcancer.net/3nlw2018/
ICARE Newsletter Winter 2018
FDA Approval of PARP Inhibitor (Lynparza) for Treatment of Advanced Breast Cancer
ICARE Newsletter Winter 2018
FDA Approval of PARP Inhibitor (Lynparza) for Treatment of Advanced Breast Cancer
On January 12, 2018, the FDA approved the first PARP Inhibitor (Lynparza) for treatment in patients with advanced breast cancer due to inherited BRCA mutations.1 This drug is already approved for certain BRCA carriers for advanced ovarian cancer. PARP inhibitors were originally developed to target the specific pathway through which cancer develops among those with …
Permanent link to this article: https://inheritedcancer.net/6nlw2018/
ICARE Newsletter Summer 2017
New Results of a PARP Inhibitor Study Among BRCA Carriers with Metastatic Breast Cancer
ICARE Newsletter Summer 2017
New Results of a PARP Inhibitor Study Among BRCA Carriers with Metastatic Breast Cancer
Over the last decade, a new class of drugs called “PARP Inhibitors” has been evaluated as a form of targeted treatment among BRCA carriers. Results were recently reported from a Phase 3 clinical trial among BRCA carriers with HER2-negative metastatic breast cancer who received two or less prior chemotherapy regimens for their metastatic disease. Study …
Permanent link to this article: https://inheritedcancer.net/4nls2017/
ICARE Newsletter Winter 2017
Newly Approved PARP-Inhibitor (Rucaparib) to Treat BRCA Carriers with Ovarian Cancer
ICARE Newsletter Winter 2017
Newly Approved PARP-Inhibitor (Rucaparib) to Treat BRCA Carriers with Ovarian Cancer
The FDA just approved another PARP inhibitor, rucaparib, for BRCA carriers with ovarian cancer who have already been treated with two or more chemotherapies. Among those with BRCA-mutant ovarian cancers, 54% had a partial or complete response to the drug with a median duration response of 9.2 months. The agency also approved a companion diagnostic …
Permanent link to this article: https://inheritedcancer.net/6nlw2017/
ICARE Newsletter Winter 2016
Potential Use of PARP-Inhibitors Among Men with Prostate Cancer Who Carry a Mutation in BRCA or Other DNA-Repair Gene
ICARE Newsletter Winter 2016
Potential Use of PARP-Inhibitors Among Men with Prostate Cancer Who Carry a Mutation in BRCA or Other DNA-Repair Gene
A recent study published in the New England Journal of Medicine suggests that PARP-Inhibitors may be of potential use in men who are no longer responding to standard treatments and carry either somatic (i.e., tumor) and/or germline (inherited) mutations in DNA-repair genes (i.e., BRCA1/2, ATM, Fanconi Anemia genes and CHEK2).1 Of 49 men with prostate …
Permanent link to this article: https://inheritedcancer.net/4nlw2016/
ICARE Newsletter Winter 2015
The First PARP-Inhibitor to Be Approved for Clinical Use in BRCA Carriers
ICARE Newsletter Winter 2015
The First PARP-Inhibitor to Be Approved for Clinical Use in BRCA Carriers
More frequently, cancer drugs are being developed to treat tumors based on their molecular make-up. PARP inhibitors are the first class of drugs specifically developed to treat BRCA-related tumors through targeting the DNA repair pathway. The PARP Inhibitors target this pathway and cause cancer cells to die while healthy cells are spared. Although PARP inhibitors …
Permanent link to this article: https://inheritedcancer.net/2nlw2015/
ICARE Newsletter Winter 2013
The Selection of Chemotherapy in BRCA Patients with Pancreatic Cancer
ICARE Newsletter Winter 2013
The Selection of Chemotherapy in BRCA Patients with Pancreatic Cancer
Some evidence suggests that individuals with BRCA mutations who develop pancreatic cancer may benefit from specific chemotherapy regimens. In a recent review of this topic, Kim et al reported on a study of 5 patients with BRCA mutations (4 BRCA2 and 1 BRCA1) who were treated with a platinum-based chemotherapy regimen.1 Of these patients, 3 …
Permanent link to this article: https://inheritedcancer.net/1nlw2013/