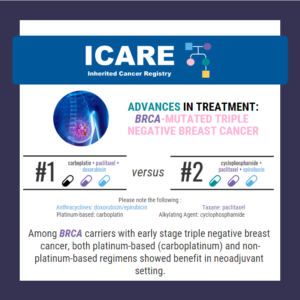
A new randomized controlled trial among BRCA1/2 carriers comparing neoadjuvant chemotherapy with olaparib versus chemotherapy alone found: These findings suggest that adding olaparib may benefit survival for BRCA1/2 carriers, even if this is not apparent when looking at pathologic response. Abraham, et al. Nat Commun. 2025;16(1):4269. PMID: 40360463. Article available at: https://pubmed.ncbi.nlm.nih.gov/40360463/. Social media post …
Care Consideration: Cancer Treatment
Permanent link to this article: https://inheritedcancer.net/nlf20259/
ICARE Social Media Post September 2025
FDA Approval Update for Patients with Neurofibromatosis Type 1
ICARE Social Media Post September 2025
FDA Approval Update for Patients with Neurofibromatosis Type 1
Big news for the NF1 community: Selumetinib is now FDA-approved for inoperable plexiform neurofibromas. A major step forward in treatment options for Neurofibromatosis Type 1! 🔗 Read the full FDA announcement at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-selumetinib-pediatric-patients-1-year-age-and-older-neurofibromatosis-type-1
Permanent link to this article: https://inheritedcancer.net/post09302025/
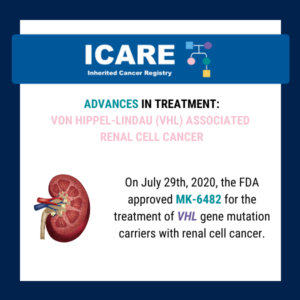
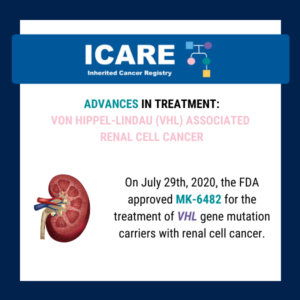
ICARE Social Media Post August 2025
Von Hippel-Lindau (VHL)-related tumors and Belzutifan treatment
ICARE Social Media Post August 2025
Von Hippel-Lindau (VHL)-related tumors and Belzutifan treatment
A new study presented at the American Society of Clinical Oncology (ASCO) annual meeting showed that Belzutifan treatment of VHL-related tumors durably shrinks tumors and may reduce the number of surgeries needed in patients with cancers associated with a von Hippel-Lindau (VHL) gene deletion or mutation. Five-year follow-up findings showed the following response rates: Learn …
Permanent link to this article: https://inheritedcancer.net/post080525/
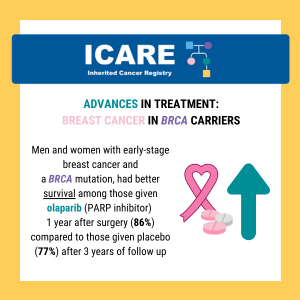
ICARE Social Media Post July 2025
Breast Cancer Treatment: BRCA Carriers
ICARE Social Media Post July 2025
Breast Cancer Treatment: BRCA Carriers
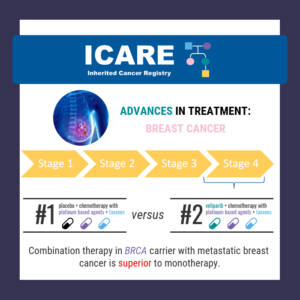
A new randomized controlled trial among BRCA1 and BRCA2 carriers comparing neoadjuvant chemotherapy with olaparib versus chemotherapy alone found: This suggests that adding olaparib may benefit survival for BRCA carriers, even if this is not apparent when looking at pathologic response. Learn more by reading the full article at: https://pmc.ncbi.nlm.nih.gov/articles/PMC12075821/ Reference: Abraham, et al. Nat …
Permanent link to this article: https://inheritedcancer.net/post070825/
ICARE Social Media Post June 2025
ATM, CHEK2, & PALB2 Carriers: Are There Differences in Cancer-associated Mortality?
ICARE Social Media Post June 2025
ATM, CHEK2, & PALB2 Carriers: Are There Differences in Cancer-associated Mortality?
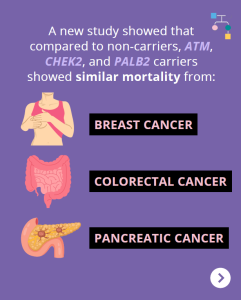
A new study showed that compared to non-carriers, ATM, CHEK2, and PALB2 carriers showed similar mortality from breast cancer, pancreatic cancer, and colorectal cancer. Other findings among BRCA1/2 carriers and Lynch Syndrome patients showed: Why is this important?These results may be reassuring for ATM, CHEK2, and PALB2 carriers, and provide additional useful information when discussing …
Permanent link to this article: https://inheritedcancer.net/post060625/
ICARE Social Media Post May 2025
Inherited Prostate Cancer: PARP Inhibitors
ICARE Social Media Post May 2025
Inherited Prostate Cancer: PARP Inhibitors
A new meta-analysis study looking at prior studies of PARP inhibitors in patients with metastatic castration-resistant prostate cancer and an inherited gene mutation showed: Learn more at: https://pubmed.ncbi.nlm.nih.gov/39848867/ Reference: Naqvi, et al. Eur Urol. 2025:S0302-2838(24)02760-X. PMID: 39848867.
Permanent link to this article: https://inheritedcancer.net/post52125/
ICARE Social Media Post April 2025
Li-Fraumeni Syndrome: Decreased Time to Second Cancer in Children Treated with Radiation
ICARE Social Media Post April 2025
Li-Fraumeni Syndrome: Decreased Time to Second Cancer in Children Treated with Radiation
A new case series of 47 children with Li-Fraumeni syndrome diagnosed with a solid cancer at or below age 16 found that: This study suggests that using radiation therapy to treat children with Li-Fraumeni syndrome and a solid tumor greatly raises the chance of getting a second cancer earlier. Therefore, it seems reasonable to only …
Permanent link to this article: https://inheritedcancer.net/post42825/
ICARE Newsletter Spring 2025
PARP Inhibitor: Relapsed BRCA-Mutated Ovarian Cancer
ICARE Newsletter Spring 2025
PARP Inhibitor: Relapsed BRCA-Mutated Ovarian Cancer
A new study (phase 3 ARIEL4 trial) to evaluate rucaparib (PARP inhibitor) versus standard-of-care chemotherapy among patients with relapsed BRCA-mutated ovarian cancer showed that median overall survival in the rucaparib group was 19.4 months versus 25.4 months in the chemotherapy group. This shows that more research is needed to figure out the most appropriate treatment …
Permanent link to this article: https://inheritedcancer.net/11nls2025/
Mar 24
ICARE Social Media Post March 2025
PARP-Inhibitor: Relapsed BRCA-Mutated Ovarian Cancer
ICARE Social Media Post March 2025
PARP-Inhibitor: Relapsed BRCA-Mutated Ovarian Cancer
A new study (phase 3 ARIEL4 trial) to evaluate rucaparib (PARP Inhibitor) versus standard-of-care chemotherapy among patients with relapsed BRCA-mutated ovarian cancer showed median overall survival in the rucaparib group was 19.4 months versus 25.4 months in the chemotherapy group. This shows that more research is needed to figure out the most appropriate treatment options …
Permanent link to this article: https://inheritedcancer.net/post32425/
ICARE Social Media Post February 2025
CHEK2: Double Mutation Carriers and Risks
ICARE Social Media Post February 2025
CHEK2: Double Mutation Carriers and Risks
There are three CHEK2 “low-risk” mutations with lower breast cancer risks: p.I157T, p.S428F, and p.T476M. A new study was conducted on how combinations of low- and regular-risk CHEK2 mutations may affect breast cancer risk. Results showed the following risks for various combinations of variants: Accompanying editorial by Dr. Rajagopal highlights: Check out the articles to …
Permanent link to this article: https://inheritedcancer.net/post21025/
ICARE Social Media Post January 2025
Risk-reducing surgeries extend survival among young BRCA carriers with breast cancer history
ICARE Social Media Post January 2025
Risk-reducing surgeries extend survival among young BRCA carriers with breast cancer history
🔬 A new study presented at the 2024 San Antonio Breast Cancer Symposium suggests that risk-reducing surgeries can improve survival for young BRCA carriers with a history of breast cancer. Among BRCA carriers with early breast cancer, risk-reducing surgeries (breast and/or ovaries) were found to: Read the full article to learn more at: https://www.healio.com/news/hematology-oncology/20241211/riskreducing-surgeries-extend-survival-among-young-brca-carriers-with-breast-cancer-history Reference: …
Permanent link to this article: https://inheritedcancer.net/post12125/
ICARE Social Media Post November 2024
BRCA1/2 Carriers with Advanced Breast Cancer
ICARE Social Media Post November 2024
BRCA1/2 Carriers with Advanced Breast Cancer
As highlighted in our latest ICARE newsletter, a new study in BRCA1/2 carriers with advanced breast cancer found: Read the full article to learn more at: https://pubmed.ncbi.nlm.nih.gov/37437366/Reference: Valenza, et al. Eur J Cancer. 2023;190:112944. PMID: 37437366. We also encourage you to read the full ICARE newsletter for other clinical and research updates at https://inheritedcancer.net/newsletters/
Permanent link to this article: https://inheritedcancer.net/icare-social-media-post-november-2024-brca1-2-carriers-with-advanced-breast-cancer/
ICARE Social Media Post November 2024
Breast Cancer After Ovarian Cancer in BRCA Carriers
ICARE Social Media Post November 2024
Breast Cancer After Ovarian Cancer in BRCA Carriers
As highlighted in the latest ICARE newsletter, a recent study evaluated breast cancer risks after ovarian cancer in BRCA1 & BRCA2 carriers. 🔍 After chemotherapy for ovarian cancer: 📊 Incidence rates were: 📈 What does this mean? Learn more at: https://tinyurl.com/yc8de3py Reference: Evans, et al. Genet Med. 2024;26(9):101172. PMID: 38847192. We also encourage you to …
Permanent link to this article: https://inheritedcancer.net/post111924/
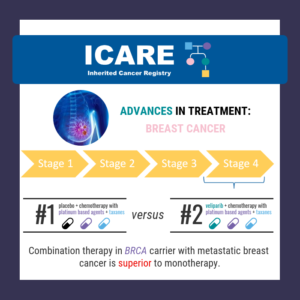
ICARE Social Media Post November 2024
BRCA Carriers with Breast Cancer: Trial to Compare PARP Inhibitors to Chemotherapy
ICARE Social Media Post November 2024
BRCA Carriers with Breast Cancer: Trial to Compare PARP Inhibitors to Chemotherapy
As highlighted in the latest ICARE newsletter, a recent trial among BRCA1/2 carriers with breast cancer comparing PARP inhibitors to standard chemotherapy (Treatment of Physician’s Choice – TPC) found that after 25.7 months of follow up: Overall survival in each group was: The % alive at 3 years was: Patients who received Olaparib for first …
Permanent link to this article: https://inheritedcancer.net/post111224/
ICARE Social Media Post October 2024
November 2024 BRCA Hybrid Event Post – Patients & Public (Nov 5th) First post
ICARE Social Media Post October 2024
November 2024 BRCA Hybrid Event Post – Patients & Public (Nov 5th) First post
Are you or someone you love affected by a BRCA gene mutation 🧬 ? This event is for you! On November 5th, Women’s College Hospital will be hosting a hybrid event entitled, BRCA: 30 Years, Discovery to Impact, to celebrate 30 years since the groundbreaking discovery of the BRCA1 and BRCA2 genes. Learn from leading …
Permanent link to this article: https://inheritedcancer.net/post101724/
ICARE Social Media Post October 2024
Double Mastectomy Not Linked To Survival Advantage
ICARE Social Media Post October 2024
Double Mastectomy Not Linked To Survival Advantage
A recent study found that in women with breast cancer, double mastectomy did NOT lead to longer survival. This is very important information for women to know as they make decisions about breast cancer surgery. Read the full article to learn more at: https://bit.ly/47a5O1b Reference: Giannakeas, et al. JAMA Oncol. 2024:e242212. PMID: 39052262.
Permanent link to this article: https://inheritedcancer.net/post100924_1/
ICARE Social Media Post October 2024
NCCN Colorectal, Endometrial, and Gastric Cancer Guidelines Update V2.2024
ICARE Social Media Post October 2024
NCCN Colorectal, Endometrial, and Gastric Cancer Guidelines Update V2.2024
The National Comprehensive Cancer Network (NCCN) released updated Genetic Familial High-Risk Assessment Colorectal, Endometrial, and Gastric Cancer guidelines on October 3rd, 2024. Updates include ⤸ Added the following to testing being considered: Personal history of colorectal or endometrial cancer at or older than age 50, and: Revised information about EPCAM gene (which has usually been …
Permanent link to this article: https://inheritedcancer.net/post100824/
ICARE Newsletter Fall 2024
Community Spotlight: The Patient and the Researcher Shares Her Uncertain Future and Lessons She’s Learned
ICARE Newsletter Fall 2024
Community Spotlight: The Patient and the Researcher Shares Her Uncertain Future and Lessons She’s Learned
By Marleah Dean Kruzel, PhD, BRCA2 Previvor When I was eight years old, my mother found a lump in her breast – barely noticeable. For a few years, I watched her undergo chemotherapy, radiation, and a prophylactic mastectomy and reconstruction. Since then, my maternal aunt and grandmother also fought breast cancer, and we learned my …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2024-community-spotlight-the-patient-and-the-researcher-shares-her-uncertain-future-and-lessons-shes-learned/
ICARE Newsletter Fall 2024
Double Mastectomy Not Linked to Survival Advantage
ICARE Newsletter Fall 2024
Double Mastectomy Not Linked to Survival Advantage
A recent study found that in females with unilateral breast cancer (meaning breast cancer on one side) who decided to have a double mastectomy had similar mortality rates to those treated with lumpectomy or unilateral mastectomy (meaning double mastectomy did NOT lead to longer survival). Findings showed that while bilateral mastectomy greatly lowered the risk …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2024-double-mastectomy-not-linked-to-survival-advantage/
ICARE Newsletter Fall 2024
BRCA1/2 Carriers: Treatment Advances
ICARE Newsletter Fall 2024
BRCA1/2 Carriers: Treatment Advances
Among BRCA1/2 carriers with advanced breast cancer, PARP inhibitors showed some activity even in patients with platinum resistant/unresponsive disease. However, the optimal delivery of platinum agents and PARP inhibitors was not clear.1 In another study of BRCA1/2 carriers with breast cancer (OlympiAD Trial), PARP inhibitors were compared to chemotherapy Treatment of Physician’s Choice (TPC), with …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2024-brca1-2-carriers-treatment-advances/
ICARE Newsletter Fall 2024
Breast Cancer After Ovarian Cancer in BRCA1/2 Carriers
ICARE Newsletter Fall 2024
Breast Cancer After Ovarian Cancer in BRCA1/2 Carriers
A recently published study reported that among females with ovarian cancer who received chemotherapy, their risk for breast cancer was lower for the next 5 years. Specifically, incidence rates were lower at 2 years (1.18%) and between 2 to 5 years (1.13%); however, incidence rates rose thereafter for BRCA1 carriers (>4% annually post 10 years). This study …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2024-breast-cancer-after-ovarian-cancer-in-brca1-2-carriers/
ICARE Newsletter Fall 2024
BRCA1/2 Carriers and Pregnancy-Related Risk
ICARE Newsletter Fall 2024
BRCA1/2 Carriers and Pregnancy-Related Risk
A recent study reported that pregnancy after breast cancer was safe for both mother and baby.1 Specifically, the researchers found that pregnancy after breast cancer was not associated with adverse maternal prognosis or fetal outcomes. Another study reported that breast cancer after pregnancy could be associated with poorer outcomes. Specifically, breast cancer diagnosed within 10 …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2024-brca1-2-carriers-and-pregnancy-related-ris/
ICARE Newsletter Spring 2024
Ask the Expert
ICARE Newsletter Spring 2024
Ask the Expert
This question was addressed by Ronald D. Alvarez, MD, MBA, Professor and Chairman of the Department of Obstetrics and Gynecology at the Vanderbilt University Medical Center in Nashville, Tennessee. He is also the current vice chair of the National Comprehensive Cancer Network (NCCN) Ovarian Cancer Treatment Guidelines and has served in multiple leadership roles in …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2024-ask-the-expert/
ICARE Newsletter Spring 2024
How Well Do Cancer Risk Management strategies Work Among BRCA Carriers
ICARE Newsletter Spring 2024
How Well Do Cancer Risk Management strategies Work Among BRCA Carriers
Several important studies were published recently on the effectiveness of risk management strategies in BRCA carriers. Specifically, a recently published study in which ICARE participants were included suggested that preventive bilateral mastectomy for BRCA carriers greatly reduced the risk of developing breast cancer by 80%.1 Additionally, study findings showed that after preventive mastectomy, the chance …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2024-how-well-do-cancer-risk-management-strategies-work-among-brca-carriers/
ICARE Newsletter Spring 2024
National Comprehensive Cancer Network (NCCN) Guideline Updates
ICARE Newsletter Spring 2024
National Comprehensive Cancer Network (NCCN) Guideline Updates
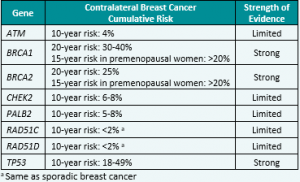
Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic Cancer – Released February 12th, 2024 (V3.2024) Check out the full guidelines by creating a FREE account at www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf Contralateral breast cancer risks in these updated guidelines: Expanded guidance about gynecologic cancers in BRCA1/2 carriers: Some highlights related to HRT include: Genetic/Familial High-Risk Assessment: Colorectal Cancer – Released …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-spring-2024-national-comprehensive-cancer-network-nccn-guideline-updates/
ICARE Featured Video March 2024
Liquid Biopsies to Guide Breast Cancer Therapies
ICARE Featured Video March 2024
Liquid Biopsies to Guide Breast Cancer Therapies
Below is a featured video from the March 2024 case conference, during which Ben Ho Park, MD, PhD discusses liquid biopsies.
Permanent link to this article: https://inheritedcancer.net/video30724/
PARP Inhibitor (Olaparib) in men with BRCA mutations and prostate cancer
A recent study found that Olaparib (Lynparza) improved survival outcomes among men with BRCA1/2 mutations and metastatic castration-resistant prostate cancer, regardless of whether the mutation was germline or somatic. This underscores the potential of targeted therapies in improving outcomes for those with inherited cancer gene mutations. Learn more at: https://ascopubs.org/doi/10.1200/JCO.23.00339 Reference: Mateo, et al. J …
Permanent link to this article: https://inheritedcancer.net/post22024/
ICARE Social Media Post February 2024
Updates to NCCN Guidelines: Genetic/Familial Breast, Ovarian, and Pancreatic Post #1
ICARE Social Media Post February 2024
Updates to NCCN Guidelines: Genetic/Familial Breast, Ovarian, and Pancreatic Post #1
The National Comprehensive Cancer Network (NCCN) just released updated Genetic/Familial Breast, Ovarian, and Pancreatic Cancer guidelines on February 12th, 2024! Updates include adding contralateral breast cancer risks for BRCA1, BRCA2, PALB2, CHEK2, and other genes to the GENE-A (Cancer Risk Management) table 🧬 You can check out the full guidelines by creating a FREE account …
Permanent link to this article: https://inheritedcancer.net/post21324/
ICARE Social Media Post February 2024 Updates to NCCN Guidelines: Genetic/Familial Breast, Ovarian, and Pancreatic Post #2
The National Comprehensive Cancer Network (NCCN) just released updated Genetic/Familial Breast, Ovarian, and Pancreatic Cancer guidelines on February 12th, 2024! Updates include expanded guidance about gynecologic cancers in BRCA1 and BRCA2, including:✓ Reproductive considerations✓ Non-surgical and surgical risk reduction✓ Salpingectomy✓ Hysterectomy considerations✓ HRT after risk-reducing removal of the ovaries You can check out the full …
Permanent link to this article: https://inheritedcancer.net/post21324_2/
Permanent link to this article: https://inheritedcancer.net/post102923/
ICARE Newsletter Fall 2023
BRCA-associated Prostate Cancers
ICARE Newsletter Fall 2023
BRCA-associated Prostate Cancers
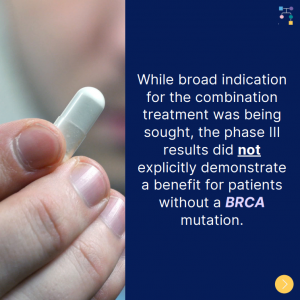
On April 28th, 2023, the FDA approved olaparib plus abiraterone acetate for first line treatment for metastatic castration-resistant prostate cancer, but only in patients whose tumors have BRCA mutations. Although a broad indication for the combination therapy was desired, concerns about the trial design were raised, and the phase III results did not explicitly show …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2023-brca-associated-prostate-cancers/
Oct 05
ICARE Newsletter Fall 2023
Healthcare Delivery
ICARE Newsletter Fall 2023
Healthcare Delivery
A recent study, based on ICARE participants, found that getting care according to guidelines depends on what the healthcare provider recommends, as well as how much trust the patient has in their care. This study shows us how important it is to find ways to improve knowledge among healthcare providers and trust in care among …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2023-healthcare-delivery/
ICARE Newsletter Fall 2023
Genes Associated with Aggressive Prostate Cancer
ICARE Newsletter Fall 2023
Genes Associated with Aggressive Prostate Cancer
A new study of almost 18,000 men with prostate cancer showed that inherited mutations in the BRCA2, ATM, and NBN genes were strongly associated with aggressive prostate cancer. Less strong associations were seen for inherited mutations in the MSH2, XRCC2, and MRE11A genes. The findings of this study suggest that knowing about inherited genes that …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2023-genes-associated-with-aggressive-prostate-cancer/
ICARE Newsletter Fall 2023
Newly released ACMG Clinical Practice Resource on CHEK2 Developed Through a Group of Worldwide Experts!
ICARE Newsletter Fall 2023
Newly released ACMG Clinical Practice Resource on CHEK2 Developed Through a Group of Worldwide Experts!
A person with a pathogenic variant in the CHEK2 gene may be at an increased risk for developing breast and other cancers. This ACMG Clinical Practice Resource, published in ACMG’s flagship journal, Genetics in Medicine, provides valuable information for healthcare providers caring for individuals with pathogenic variants in the CHEK2gene. This new ACMG Practice Resource …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2023-newly-released-acmg-clinical-practice-resource-on-chek2-developed-through-a-group-of-worldwide-experts/
ICARE Newsletter Fall 2023
National Comprehensive Cancer Network (NCCN) Guidelines Updates
ICARE Newsletter Fall 2023
National Comprehensive Cancer Network (NCCN) Guidelines Updates
Check out the full NCCN guidelines by creating a FREE account at www.nccn.org Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic CancerReleased August 28th, 2023 (V1.2024) › Transgender, Non-Binary, and Gender Diverse Individuals: NEW section on care (Page 63-66, TNBGD-1 to 4)› Li-Fraumeni Syndrome: Significant updates to content (risks and care) (Pages 57-60, LIFR-A): Table added …
Permanent link to this article: https://inheritedcancer.net/icare-newsletter-fall-2023-national-comprehensive-cancer-network-nccn-guidelines-updates/
ICARE Social Media Post August 2023
FDA Approval Post – Approves Niraparib
ICARE Social Media Post August 2023
FDA Approval Post – Approves Niraparib
A critical step forward in cancer care! The FDA has approved the use of niraparib and abiraterone acetate with prednisone in treating patients with BRCA-mutated castration-resistant prostate cancer. The green light comes backed by the robust efficacy data from the MAGNITUDE trial. Read more about the FDA approval at https://tinyurl.com/dzdvtm9m Learn more about the MAGNITUDE …
Permanent link to this article: https://inheritedcancer.net/post82423/
ICARE Featured Video August 2023
SELECT: Using a Gene Expression-driven Algorithm to Prioritize Competing Treatment Options for BRCA Carriers with Breast Cancer
ICARE Featured Video August 2023
SELECT: Using a Gene Expression-driven Algorithm to Prioritize Competing Treatment Options for BRCA Carriers with Breast Cancer
Below is a featured video from the August 2023 case conference, during which Sheila Rajagopal, MD, MPH, MSc from the National Cancer Institute presents on using a gene expression-driven algorithm to prioritize competing treatment options for BRCA carriers with breast cancer.
Permanent link to this article: https://inheritedcancer.net/video81023/
ICARE Social Media Post August 2023
New Article – Cancer Risk Management
ICARE Social Media Post August 2023
New Article – Cancer Risk Management
A recent study, which 𝗶𝗻𝗰𝗹𝘂𝗱𝗲𝗱 𝗜𝗖𝗔𝗥𝗘 𝗽𝗮𝗿𝘁𝗶𝗰𝗶𝗽𝗮𝗻𝘁𝘀, found that getting care according to guidelines depends on: Therefore, we need ways to improve knowledge among healthcare providers and trust in care among patients! Use this link to learn more: https://tinyurl.com/n26m4zys Reference: Dean, et al. Genet Med. 2023;100945. PMID: 37515473.
Permanent link to this article: https://inheritedcancer.net/post80723/
ICARE Social Media Post June 2023
FDA Advisory Committee Recommendation: Olaparib for Prostate Cancer Treatment
ICARE Social Media Post June 2023
FDA Advisory Committee Recommendation: Olaparib for Prostate Cancer Treatment
Olaparib plus abiraterone acetate is recommended as the first line treatment for metastatic castration-resistant prostate cancer, but only in patients whose tumors have BRCA mutations. Although a broad indication for the combination therapy was desired, concerns about the trial design were raised, and the phase III results did not explicitly show that patients without a …
Permanent link to this article: https://inheritedcancer.net/post61623/
ICARE Social Media Post May 2023
Cancer Care: Transgender and Gender-Diverse Persons
ICARE Social Media Post May 2023
Cancer Care: Transgender and Gender-Diverse Persons
Did you know that transgender and gender-diverse persons face unique challenges that can influence cancer risk and outcomes? For instance, these individuals face barriers to healthcare access and inequities in treatment, with healthcare providers lacking knowledge about the health needs of this population. Solutions are needed to offer the best care for these individuals. Use …
Permanent link to this article: https://inheritedcancer.net/post52223/
ICARE Social Media Post March 2023
Treatment for PALB2-associated breast cancer with Talazoparib
ICARE Social Media Post March 2023
Treatment for PALB2-associated breast cancer with Talazoparib
According to a recent Phase II study, Talazoparib may benefit patients with PALB2-associated breast cancer. Findings showed that Talazoparib appeared effective and safe in certain patients with advanced disease. Read the full article at this link: https://www.nature.com/articles/s43018-022-00439-1 Reference: Gruber et al. Nat Cancer. 2022;3(10):1181-1191. PMID: 36253484.
Permanent link to this article: https://inheritedcancer.net/post32723/
ICARE Social Media Post November 2022
Guidelines for Hereditary Breast Cancer Treatment
ICARE Social Media Post November 2022
Guidelines for Hereditary Breast Cancer Treatment
The Expert Panel Consensus, chaired by the Society of Surgical Oncology with the American Society of Clinical Oncology and the American Society of Radiation Oncology, published new recommendations for hereditary breast cancer treatment. Learn more at https://pubmed.ncbi.nlm.nih.gov/32898012/ Reference: Corso & Magnoni. Eur J Cancer Prev. 2021;30(4):311-314. PMID: 32898012.
Permanent link to this article: https://inheritedcancer.net/post110822/
ICARE Social Media Post August 2022
Pancreatic Cancer Treatment
ICARE Social Media Post August 2022
Pancreatic Cancer Treatment
A new study reports that maintenance treatment with Olaparib may benefit BRCA1/2 carriers with pancreatic cancer. These findings demonstrated:long-term survival was more commontime to subsequent therapy was prolongedRead the full article at the link: https://ascopubs.org/doi/pdf/10.1200/JCO.21.01604Reference: Kindler et al. J Clin Oncol. 2022; JCO2101604. PMID: 35834777.
Permanent link to this article: https://inheritedcancer.net/post80522/
ICARE Social Media Post June 2022
Refractory Pancreatic or Biliary Cancer
ICARE Social Media Post June 2022
Refractory Pancreatic or Biliary Cancer
A recent small study suggests that immunotherapy may be beneficial for patients with refractory pancreatic or biliary cancer who have inherited homologous recombination deficiency (HRD) genes, BRCA1, BRCA2, and RAD51C.Check out the full article to learn more at 👇https://jamanetwork.com/journals/jamaoncology/article-abstract/2791557Reference: Terrero et al. JAMA Oncol. 2022 Apr:e220611. doi:10.1001/jamaoncol.2022.0611. PMID: 35446342.
Permanent link to this article: https://inheritedcancer.net/post61322/
Permanent link to this article: https://inheritedcancer.net/post51022/
ICARE Social Media Post April 2022
BRCA1/2 Carriers with Breast Cancer: Olaparib & Survival
ICARE Social Media Post April 2022
BRCA1/2 Carriers with Breast Cancer: Olaparib & Survival
A new study found that adjuvant olaparib significantly extended survival in BRCA1/2 carriers with HER2-negative high-risk early-stage breast cancer. Learn more at the following link: https://www.healio.com/news/hematology-oncology/20220323/adjuvant-olaparib-prolongs-survival-for-certain-patients-with-early-breast-cancer
Permanent link to this article: https://inheritedcancer.net/post41922/
Permanent link to this article: https://inheritedcancer.net/post40822/
ICARE Social Media Post March 2022
FDA Approves Olaparib for Adjuvant Treatment of High-risk Early Breast Cancer
ICARE Social Media Post March 2022
FDA Approves Olaparib for Adjuvant Treatment of High-risk Early Breast Cancer
On March 11th, the Food and Drug Administration (FDA) approved olaparib (Lynparza) for the adjuvant treatment of BRCA1/2 carriers with human epidermal growth factor receptor 2 (HER2)-negative, high-risk early breast cancer who have been treated with neoadjuvant or adjuvant chemotherapy.Learn more at 👇https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-olaparib-adjuvant-treatment-high-risk-early-breast-cancer
Permanent link to this article: https://inheritedcancer.net/post31522/
Permanent link to this article: https://inheritedcancer.net/post30122/
Jan 21
ICARE Social Media Post January 2022
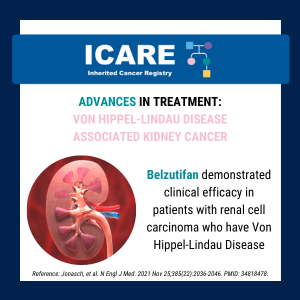
Belzutifan in Von Hippel-Lindau Disease Patients
ICARE Social Media Post January 2022
Belzutifan in Von Hippel-Lindau Disease Patients
Belzutifan demonstrated clinical efficacy in patients with renal cell carcinoma who have Von Hippel-Lindau Disease. Learn more by reading the full The New England Journal of Medicine article at 👇https://www.nejm.org/doi/full/10.1056/NEJMoa2103425Reference: Jonasch, et al. N Engl J Med. 2021 Nov 25;385(22):2036-2046. PMID: 34818478.
Permanent link to this article: https://inheritedcancer.net/post12122/
Permanent link to this article: https://inheritedcancer.net/post10422/
ICARE Social Media Post December 2021
Fall 2021 Ask the Expert
ICARE Social Media Post December 2021
Fall 2021 Ask the Expert
In every ICARE newsletter we give our participants the opportunity to have a question addressed by an expert in the field. In the latest edition, Dr. Kerry Schaffer discusses the use of PARP inhibitors to treat inherited forms of prostate cancer.Check out Dr. Schaffer’s full response at 👇https://inheritedcancer.net/newsletters/
Permanent link to this article: https://inheritedcancer.net/post122821/
ICARE Social Media Post December 2021
FDA Approves Belzutifan for Cancers Associated With von Hippel-Lindau Disease
ICARE Social Media Post December 2021
FDA Approves Belzutifan for Cancers Associated With von Hippel-Lindau Disease
On August 13th, the FDA approved the use of belzutifan (Welireg) for adult patients with von Hippel-Lindau (VHL) disease who require therapy for associated renal cell carcinoma, central nervous system hemangioblastomas, or pancreatic neuroendocrine tumors not requiring immediate surgery. For more information, check out The ASCO Post article at 👇https://ascopost.com/news/august-2021/fda-approves-belzutifan-for-cancers-associated-with-von-hippel-lindau-disease/?utm_source=TAP-EN-081321&utm_medium=email&utm_term=49cf1c97d48c2cf8231827e3bcb15769
Permanent link to this article: https://inheritedcancer.net/post121021/
ICARE Social Media Post December 2021
FDA Approves PARP Inhibitor (Olaparib) Treatment for some BRCA carriers with Early Stage Breast Cancer
ICARE Social Media Post December 2021
FDA Approves PARP Inhibitor (Olaparib) Treatment for some BRCA carriers with Early Stage Breast Cancer
FDA granted priority review to Olaparib for adjuvant treatment of certain patients with high-risk breast cancer. This designation applies to use of the agent by patients with BRCA-mutated, HER2-negative, high-risk early breast cancer who receive chemotherapy before or after surgery. For additional information, visit: https://tinyurl.com/healioFDAapproval
Permanent link to this article: https://inheritedcancer.net/post120621/
Permanent link to this article: https://inheritedcancer.net/post81621/
ICARE Featured Video August 2021
Updates to National Comprehensive Cancer Network (NCCN) Guidelines
ICARE Featured Video August 2021
Updates to National Comprehensive Cancer Network (NCCN) Guidelines
Below you may watch a featured video from the August 2021 ICARE Genetics Case Conferences outlining updates to National Comprehensive Cancer Network (NCCN) guidelines.
Permanent link to this article: https://inheritedcancer.net/video81221_2/
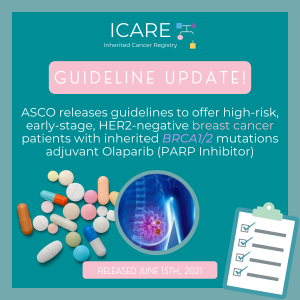
ICARE Social Media Post August 2021
ASCO Guideline Update: Olaparib for Breast Cancer
ICARE Social Media Post August 2021
ASCO Guideline Update: Olaparib for Breast Cancer
For additional information, read the updated American Society of Clinical Oncology (ASCO) recommendation (released June 15th, 2021) at the following link: https://www.asco.org/practice-patients/guidelines/breast-cancer?intcmp=ws_ascoorg_gdlns_hereditarybreastcancer_site_pressrelease_061621____#/143725
Permanent link to this article: https://inheritedcancer.net/post81021/
Permanent link to this article: https://inheritedcancer.net/post71621/
Permanent link to this article: https://inheritedcancer.net/post70921/
ICARE Social Media Post July 2021
Advances in Pancreatic Cancer Treatment: PALB2 & BRCA1/2
ICARE Social Media Post July 2021
Advances in Pancreatic Cancer Treatment: PALB2 & BRCA1/2
For more information, view the article at the following link below: https://ascopubs.org/doi/abs/10.1200/JCO.21.00003 You may also read the ASCO post article at: https://ascopost.com/news/may-2021/maintenance-rucaparib-in-patients-with-platinum-sensitive-pancreatic-cancer-and-germline-or-somatic-brca1-brca2-or-palb2-variants/?utm_source=TAP%2DEN%2D051221%2DTrending%5FLymphoma&utm_medium=email&utm_term=49cf1c97d48c2cf8231827e3bcb15769
Permanent link to this article: https://inheritedcancer.net/post70621/
Permanent link to this article: https://inheritedcancer.net/post61121/
Permanent link to this article: https://inheritedcancer.net/post52821/
Permanent link to this article: https://inheritedcancer.net/post51821/
Permanent link to this article: https://inheritedcancer.net/post22321/
ICARE Newsletter Winter 2021
Inherited Cancer Treatment: Updates and Relevant Policies
ICARE Newsletter Winter 2021
Inherited Cancer Treatment: Updates and Relevant Policies
Over the last several months, the American Society of Clinical Oncology published a number of guidelines related to the use of PARP inhibitors among those with BRCA-associated cancers, including guidelines focused on ovarian cancer,1 metastatic pancreatic cancer,2 and breast cancer.3 Additionally, costs of drugs also have great potential to influence policy, highlighting the importance of …
Permanent link to this article: https://inheritedcancer.net/7nlw2021/
ICARE Social Media Post February 2021
ICARE Winter 2021 Newsletter
ICARE Social Media Post February 2021
ICARE Winter 2021 Newsletter
The ICARE Winter 2021 Newsletter is now available! Check out this latest edition for recent research and clinical updates, a Q&A with a genomics expert, and an inspiring community spotlight piece. You can read the newsletter by visiting: https://inheritedcancer.net/wp-content/uploads/ICARE-2021-Winter-Newsletter.pdf. Please feel free to share with family members, friends, and/or your healthcare providers.
Permanent link to this article: https://inheritedcancer.net/post20921/
Permanent link to this article: https://inheritedcancer.net/post112720/
ICARE Social Media Post November 2020
Clinical Trial Participation Powers Patient’s Positive Attitude
ICARE Social Media Post November 2020
Clinical Trial Participation Powers Patient’s Positive Attitude
Brooke Thomas has leaned on 12 years of experience as a medical social worker and found ways to stay positive and upbeat through it all – and she has a lot to be positive about these days, thanks to an amazing response to her treatment as part of a clinical trial at Vanderbilt-Ingram Cancer Center. …
Permanent link to this article: https://inheritedcancer.net/post110620/
ICARE Social Media Post October 2020
New ASCO Guidelines On Use Of PARP Inhibitors To Manage Ovarian Cancer
ICARE Social Media Post October 2020
New ASCO Guidelines On Use Of PARP Inhibitors To Manage Ovarian Cancer
New guidelines for the use of PARP inhibitors to treat ovarian cancer among those with BRCA1 or BRCA2 mutations were published through the American Society of Clinical Oncology (ASCO) to guide providers about the role of this class of drugs in the management of this type of cancer. Link to the guidelines are available at: …
Permanent link to this article: https://inheritedcancer.net/post101320/
ICARE Social Media Post October 2020
Addition of Veliparib to Carboplatin/Paclitaxel in Previously Treated Patients With BRCA-Mutated Advanced Breast Cancer
ICARE Social Media Post October 2020
Addition of Veliparib to Carboplatin/Paclitaxel in Previously Treated Patients With BRCA-Mutated Advanced Breast Cancer
Among BRCA carriers with metastatic breast cancer, the combination of veliparib AND chemotherapy with platinum-based agents (carboplatin) and taxanes (paclitaxel) led to a longer duration of progression free survival (disease that did not progress), compared to those treated with ONLY chemotherapy. To read the full article visit: https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(20)30447-2/fulltext [This finding was previously outlined last year …
Permanent link to this article: https://inheritedcancer.net/post100620/
Permanent link to this article: https://inheritedcancer.net/post92220/
Permanent link to this article: https://inheritedcancer.net/post91820/
ICARE Social Media Post September 2020
ICARE Summer 2020 Newsletter
ICARE Social Media Post September 2020
ICARE Summer 2020 Newsletter
The ICARE Summer 2020 Newsletter is now available! Check out this latest edition for recent research and clinical updates as well as a Q&A with a nationally renowned clinical geneticist from Vanderbilt-Ingram Cancer Center. You can read the newsletter by visiting: https://inheritedcancer.net/wp-content/uploads/ICARE-2020-Summer-Newsletter.pdf Please feel free to share with family members, friends, and/or your …
Permanent link to this article: https://inheritedcancer.net/post91620/
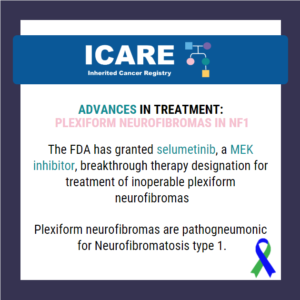
ICARE Newsletter Summer 2020
Treatment Advances Among Those with Neurofibromatosis Type 1
ICARE Newsletter Summer 2020
Treatment Advances Among Those with Neurofibromatosis Type 1
There continue to be ongoing advances in treatment studies among those with inherited cancer gene mutations, which are rapidly being followed by FDA approval for specific cancer treatments. Select studies and advances are summarized below: Neurofibromatosis Type 1 (NF1): The FDA granted selumetinib (a MEK inhibitor) breakthrough therapy designation for treatment of inoperable plexiform neurofibromas.
Permanent link to this article: https://inheritedcancer.net/8nls2020/
ICARE Newsletter Summer 2020
Treatment Advances Among Those with Lynch Syndrome
ICARE Newsletter Summer 2020
Treatment Advances Among Those with Lynch Syndrome
There continue to be ongoing advances in treatment studies among those with inherited cancer gene mutations, which are rapidly being followed by FDA approval for specific cancer treatments. Select studies and advances are summarized below: Lynch Syndrome: Colorectal Cancer: Among patients with MSI-H or MMR-deficient colorectal cancers (frequently seen among those with Lynch syndrome), pembrolizumab …
Permanent link to this article: https://inheritedcancer.net/7nls2020/
ICARE Newsletter Summer 2020
Treatment Advances Among Those with Von-Hippel Lindau (VHL) Disease
ICARE Newsletter Summer 2020
Treatment Advances Among Those with Von-Hippel Lindau (VHL) Disease
There continue to be ongoing advances in treatment studies among those with inherited cancer gene mutations, which are rapidly being followed by FDA approval for specific cancer treatments. Select studies and advances are summarized below: Von-Hippel Lindau (VHL) Disease: Among patients with VHL-associated clear cell renal cell carcinoma (RCC), a recent study suggested potential benefit …
Permanent link to this article: https://inheritedcancer.net/9nls2020/
ICARE Newsletter Summer 2020
Treatment Advances Among BRCA1/2 Carriers
ICARE Newsletter Summer 2020
Treatment Advances Among BRCA1/2 Carriers
There continue to be ongoing advances in treatment studies among those with inherited cancer gene mutations, which are rapidly being followed by FDA approval for specific cancer treatments. Select studies and advances are summarized below: BRCA1/2 Carriers: Breast Cancer: For those with later stage or metastatic breast cancer, the FDA currently has approvals for the use …
Permanent link to this article: https://inheritedcancer.net/2nls2020/
ICARE Social Media Post July 2020
BRCA1/2 and Other Gene Carriers with Breast Cancer Don’t Always Receive Recommended Treatment
ICARE Social Media Post July 2020
BRCA1/2 and Other Gene Carriers with Breast Cancer Don’t Always Receive Recommended Treatment
BRCA1/2 and other gene mutation carriers with early stage breast cancer are not always receiving cancer treatment as recommended by national guidelines. Even though more and more people have been tested for hereditary cancer over the years, using this information accurately to guide treatment has not been as successful. These findings highlight the need for …
Permanent link to this article: https://inheritedcancer.net/post71020/
ICARE Social Media Post June 2020
Advances in Treatment for Colorectal Cancer
ICARE Social Media Post June 2020
Advances in Treatment for Colorectal Cancer
On June 29, 2020, the FDA approved the use of Keytruda (pembrolizumab), as first-line treatment in unresectable or metastatic, microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) colorectal cancer. Among people with Lynch syndrome, the risk for colorectal cancer (which often has microsatellite instability and/or mismatch-repair deficiency) is raised. Keytruda (pembrolizumab) showed to double the progression-free …
Permanent link to this article: https://inheritedcancer.net/post63020/
ICARE Social Media Post June 2020
Advances in Treatment for BRCA-Mutated Triple Negative Breast Cancer
ICARE Social Media Post June 2020
Advances in Treatment for BRCA-Mutated Triple Negative Breast Cancer
In a study of 914 women with different breast cancer subtypes, overall pathologic complete response rates were: Higher in those with BRCA1/2 mutations (60.4% versus 46.7%) No differences were seen in those with mutations in other inherited cancer genes Among patients with triple-negative breast cancer, BRCA1/2 mutations had highest response rates to treatment in both …
Permanent link to this article: https://inheritedcancer.net/post61920/
ICARE Social Media Post June 2020
Advances in BRCA1/2 Breast Cancer Treatment
ICARE Social Media Post June 2020
Advances in BRCA1/2 Breast Cancer Treatment
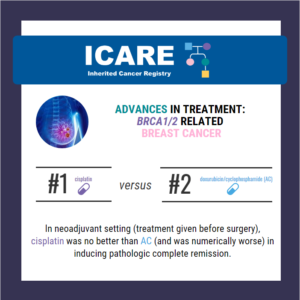
Through a randomized phase 2 study (called the INFORM trial) among BRCA1/2 carriers with breast cancer, cisplatin was no better in inducing pathologic complete remission compared to AC. The pathologic complete remission rate was 18% for cisplatin and 26% for AC. Cisplatin is not better than other chemotherapy for induction therapy for breast cancers in …
Permanent link to this article: https://inheritedcancer.net/post61220/
ICARE Social Media Post June 2020
Advances in Treatment for Pancreatic Cancer: Cisplatin + Gemcitabine
ICARE Social Media Post June 2020
Advances in Treatment for Pancreatic Cancer: Cisplatin + Gemcitabine
In BRCA1/2 or PALB2 carriers with stage 3 or 4 pancreatic cancer, the combination of cisplatin + gemtricitabine with veliparib (a PARP inhibitor), did NOT seem to provide additional benefit over cisplatin + gemtricitabine alone. Through this phase 2 randomized control trial, response rates in both treatment arms were high with similar overall survival rates. …
Permanent link to this article: https://inheritedcancer.net/post60520/
Permanent link to this article: https://inheritedcancer.net/post52920/
ICARE Social Media Post May 2020
Advances in Treatment for Metastatic Prostate Cancer: Olaparib
ICARE Social Media Post May 2020
Advances in Treatment for Metastatic Prostate Cancer: Olaparib
On May 19, 2020 the FDA approved the use of olaparib (Lynparza) as treatment in BRCA and other gene carriers (homologous recombination repair genes) with metastatic castration-resistant prostate cancer who have been treated with enzalutamide or abiraterone. Link to full article: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-olaparib-hrr-gene-mutated-metastatic-castration-resistant-prostate-cancer
Permanent link to this article: https://inheritedcancer.net/post52220/
ICARE Social Media Post May 2020
Advances in Treatment for Metastatic Prostate Cancer: Rucaparib
ICARE Social Media Post May 2020
Advances in Treatment for Metastatic Prostate Cancer: Rucaparib
On May 15, 2020 the FDA approved the use of rucaparib (Rubraca) as treatment in BRCA carriers with metastatic castration-resistant prostate cancer who have been treated with androgen receptor-directed therapy and a taxane-based chemotherapy. Link to full article: https://www.fda.gov/drugs/fda-grants-accelerated-approval-rucaparib-brca-mutated-metastatic-castration-resistant-prostate
Permanent link to this article: https://inheritedcancer.net/post51920/
ICARE Social Media Post May 2020
Advances in Ovarian Cancer Treatment for BRCA1/2 Carriers: Olaparib & bevacizumab
ICARE Social Media Post May 2020
Advances in Ovarian Cancer Treatment for BRCA1/2 Carriers: Olaparib & bevacizumab
On May 8, 2020 the FDA approved the use of olaparib (Lynparza) as first-line maintenance treatment in BRCA1/2 carriers (deleterious or suspected deleterious mutations) and/or a genomic instability, with advanced epithelial ovarian, fallopian tube or primary peritoneal cancer who are in complete or partial response to first-line platinum-based chemotherapy. Link to full article: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-olaparib-plus-bevacizumab-maintenance-treatment-ovarian-fallopian-tube-or-primary
Permanent link to this article: https://inheritedcancer.net/post51220/
ICARE Social Media Post May 2020
Advances in Ovarian Cancer Treatment for BRCA1/2 Carriers: Olaparib
ICARE Social Media Post May 2020
Advances in Ovarian Cancer Treatment for BRCA1/2 Carriers: Olaparib
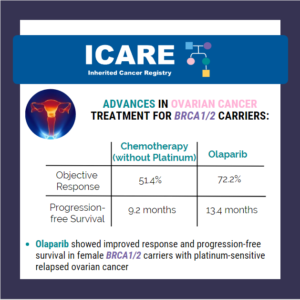
In recognition of World Ovarian Cancer Day, we’d like to share some exciting results from a study of women with ovarian cancer and a BRCA mutation: In a recent phase III trial, olaparib (PARP inhibitor) showed improved response and progression-free survival compared with chemotherapy (without platinum) in BRCA carriers with platinum-sensitive relapsed ovarian cancer who …
Permanent link to this article: https://inheritedcancer.net/post50820/
ICARE Social Media Post May 2020
Advances in Treatment for Metastatic Prostate Cancer: Olaparib
ICARE Social Media Post May 2020
Advances in Treatment for Metastatic Prostate Cancer: Olaparib
Findings from a recent study showed that olaparib (PARP inhibitor) significantly improved progression-free survival in patients with BRCA1, BRCA2, or ATM genetic alterations. Benefits were also more broadly seen among patients with homologous recombination repair gene defects. Link to full article: https://www.nejm.org/doi/full/10.1056/NEJMoa1911440 Check out the ASCO post article at: https://www.ascopost.com/news/may-2020/olaparib-for-patients-with-mcrpc-and-homologous-recombination-repair-gene-alterations/
Permanent link to this article: https://inheritedcancer.net/post50620/
ICARE Social Media Post April 2020
Advances in Treatment for Ovarian Cancer in BRCA1/2 Carriers: Niraparib
ICARE Social Media Post April 2020
Advances in Treatment for Ovarian Cancer in BRCA1/2 Carriers: Niraparib
On April 29, 2020 the FDA approved the use of niraparib (Zeluja) as first-line maintenance treatment in BRCA1/2 carriers with advanced ovarian cancer! More details available at: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-niraparib-first-line-maintenance-advanced-ovarian-cancer
Permanent link to this article: https://inheritedcancer.net/post42920/
ICARE Social Media Post April 2020
Treatment Patterns in Women with Inherited Breast Cancer
ICARE Social Media Post April 2020
Treatment Patterns in Women with Inherited Breast Cancer
Results from a population-based study of over 20,000 women from SEER registries of Georgia and California diagnosed with stage 0 to stage III breast cancer between 2014 and 2016 tested for inherited breast cancer were recently published. Findings suggest that women who test positive for certain mutations receive certain patterns of treatment. These patterns of …
Permanent link to this article: https://inheritedcancer.net/post41020/
ICARE Social Media Post March 2020
Cancer Treatment in Childhood and Treatment-Associated Polyposis
ICARE Social Media Post March 2020
Cancer Treatment in Childhood and Treatment-Associated Polyposis
Treatment-associated polyposis (TAP) should be considered in patients who develop many colon polyps after treatment for a childhood or young adulthood cancer but do not have an identified mutation in a hereditary cancer gene. A recent study reported that 35% of patients with TAP developed over 50 colorectal polyps, and 94% had multiple types of …
Permanent link to this article: https://inheritedcancer.net/post32720/
ICARE Social Media Post February 2020
Advances in Treatment: Plexiform Neurofibromas in NF1
ICARE Social Media Post February 2020
Advances in Treatment: Plexiform Neurofibromas in NF1
The FDA has granted a breakthrough therapy designation to selumetinib, a MEK inhibitor, for treatment of inoperable plexiform neurofibromas. These types of neurofibromas are almost exclusively seen in individuals with neurofibromatosis type 1 (NF1). These plexiform neurofibromas are benign tumors on the nerve sheaths and can develop anywhere in the body. These tumors typically cause …
Permanent link to this article: https://inheritedcancer.net/post22820/
ICARE Social Media Post February 2020
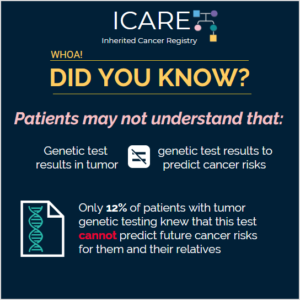
Genetic Testing in Tumors versus Genetic Testing for Cancer Risk
ICARE Social Media Post February 2020
Genetic Testing in Tumors versus Genetic Testing for Cancer Risk
There are different types of genetic/DNA tests offered to patients with cancer: 1) Tumor tests, mainly done to guide cancer treatment. 2) Blood or saliva tests (on normal DNA that individual was born with) to identify inherited cancer predisposition, which may also guide cancer treatment in some instances. Genetic testing of the tumor detects mutations …
Permanent link to this article: https://inheritedcancer.net/post22620/
ICARE Newsletter Winter 2020
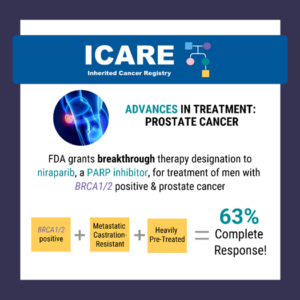
Treatment Advances Among Those with Inherited Prostate Cancer Predisposition
ICARE Newsletter Winter 2020
Treatment Advances Among Those with Inherited Prostate Cancer Predisposition
A recent study reported a high complete response rate among men with a BRCA1/2 mutation with metastatic, castration-resistant prostate cancer who were treated with niraparib (a PARP inhibitor) of 63% compared to 17% in the non-BRCA1/2 group.1 Based on this data, the Federal Drug Administration (FDA) granted breakthrough therapy designation to niraparib on October 3, …
Permanent link to this article: https://inheritedcancer.net/3nlw2020/
ICARE Newsletter Winter 2020
Treatment Advances Among Those with Inherited Pancreatic Cancer Predisposition
ICARE Newsletter Winter 2020
Treatment Advances Among Those with Inherited Pancreatic Cancer Predisposition
Results from a recent study showed olaparib (a PARP inhibitor) nearly doubled the progression-free survival in BRCA1/2 carriers with metastatic pancreatic cancer.1 Based on this data, the FDA approved the use of olaparib as a first-line maintenance treatment in BRCA1/2 carriers with metastatic, platinum-sensitive pancreatic cancer. This represents another treatment advance in pancreatic cancer and …
Permanent link to this article: https://inheritedcancer.net/2nlw2020/
ICARE Social Media Post January 2020
Advances in Treatment for Lynch Syndrome-Related Endometrial Cancer
ICARE Social Media Post January 2020
Advances in Treatment for Lynch Syndrome-Related Endometrial Cancer
A recent phase II study showed increased response to an immunotherapy drug (avelumab), in women with endometrial cancer with mismatch repair deficiency. Among women with Lynch Syndrome, the risk for endometrial cancer (which often has mismatch repair deficiency) is raised. This new study shows promising treatment options for women with endometrial cancer and Lynch syndrome …
Permanent link to this article: https://inheritedcancer.net/13020/
ICARE Social Media Post January 2020
Celebrating 10 Years of ICARE
ICARE Social Media Post January 2020
Celebrating 10 Years of ICARE
Happy New Year! 2020 represents a decade for ICARE We are celebrating 10 years of research, education, and engagement, through which we have enrolled nearly 3500 participants, including over 2000 gene mutation carriers, disseminated 15 newsletters, led and collaborated on multiple research projects, and impacted individuals affected by inherited cancer predisposition all over the …
Permanent link to this article: https://inheritedcancer.net/post1920/
ICARE Social Media Post January 2020
Advances in Treatment for Pancreatic Cancer in BRCA Carriers
ICARE Social Media Post January 2020
Advances in Treatment for Pancreatic Cancer in BRCA Carriers
The FDA approved the use of olaparib, a PARP inhibitor, as first-line maintenance treatment in BRCA1/2 carriers with metastatic, platinum-sensitive, pancreatic cancer. Platinum-sensitive cancer is a cancer that responds to treatment with drugs that contain the metal platinum, such as carboplatin or cisplatin. Olaparib showed to nearly double the progression-free survival in BRCA1/2 carriers with …
Permanent link to this article: https://inheritedcancer.net/post1320/
ICARE Social Media Post December 2019
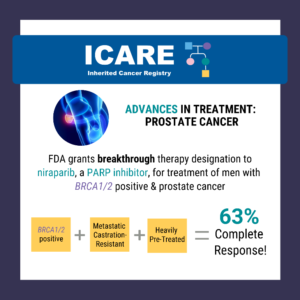
Evaluation of PARP Inhibitors in BRCA-Associated Prostate Cancer
ICARE Social Media Post December 2019
Evaluation of PARP Inhibitors in BRCA-Associated Prostate Cancer
The FDA granted breakthrough therapy designation to niraparib (a PARP inhibitor) for the treatment of men with BRCA1/2 positive, metastatic castration-resistant, and heavily pre-treated prostate cancer. Results from a recent study show a 63% complete response rate in men with BRCA1/2 positive, metastatic castration-resistant prostate cancer treated with niraparib compared to 17% in the non- …
Permanent link to this article: https://inheritedcancer.net/post122019/
ICARE Featured Video December 2019
NCCN Genetic/Familial Breast, Ovarian, and Pancreatic Guidelines
ICARE Featured Video December 2019
NCCN Genetic/Familial Breast, Ovarian, and Pancreatic Guidelines
Below you may watch a featured video from the December 2019 Genetics Case Conference, which outlined updates to the National Comprehensive Cancer Network (NCCN) guidelines.
Permanent link to this article: https://inheritedcancer.net/video121219/
ICARE Social Media Post December 2019
Patient Reported Outcomes In A Study of PARP Inhibitors in BRCA Carriers with Metastatic Breast Cancer
ICARE Social Media Post December 2019
Patient Reported Outcomes In A Study of PARP Inhibitors in BRCA Carriers with Metastatic Breast Cancer
Did you know? PROs are impacting treatment advances in metastatic breast cancer. Olaparib increased progression-free survival among BRCA carriers with metastatic HER2- breast cancer. Thanks to patient reported outcomes, a new study now suggests it also improved patients’ quality of life! Check it out the new article published in October 2019 directly at https://www.ncbi.nlm.nih.gov/pubmed/31446213!
Permanent link to this article: https://inheritedcancer.net/post12819/
ICARE Social Media Post October 2019
Advances in Treatment for Advanced Breast Cancer in BRCA Carriers
ICARE Social Media Post October 2019
Advances in Treatment for Advanced Breast Cancer in BRCA Carriers
Monotherapy with PARP inhibitors is FDA-approved for patients with metastatic breast cancer with BRCA mutations. BUT, does adding additional drugs (called ‘combination therapy’) help? In BRCA carriers with metastatic breast cancer, the combination of veliparib AND chemotherapy with platinum-based agents (carboplatin) and taxanes (paclitaxel) led to a longer duration of progression free survival (disease that …
Permanent link to this article: https://inheritedcancer.net/post101819/
ICARE Social Media Post October 2019
Advances in Early Stage Breast Cancer Treatment for BRCA Carriers
ICARE Social Media Post October 2019
Advances in Early Stage Breast Cancer Treatment for BRCA Carriers
New benefits from a PARP inhibitor, talazoparib, among BRCA carriers with early stage breast cancer. The current FDA approvals for the use of PARP inhibitors is limited to women with metastatic (stage IV) breast cancer. These drugs are being tested in early stage breast cancer to shrink down the tumor (called neoadjuvant treatment) before surgery …
Permanent link to this article: https://inheritedcancer.net/post101519/
ICARE Newsletter Summer 2019
Ovarian Cancer Treatment Advances for BRCA1/2 Carriers
ICARE Newsletter Summer 2019
Ovarian Cancer Treatment Advances for BRCA1/2 Carriers
A recently reported study of women with ovarian cancer and homologous recombination deficiency (HRD) who received a PARP inhibitor (niraparib) as fourth line or later treatment showed potential clinical benefit. Specifically, median overall survival after treatment was 19 months in the HRD-positive group (including those with BRCA1/2 mutations) compared to 15.5 months in the HRD-negative …
Permanent link to this article: https://inheritedcancer.net/4nls2019/
ICARE Newsletter Summer 2019
Pancreatic Cancer Treatment Advances for BRCA1/2 Carriers
ICARE Newsletter Summer 2019
Pancreatic Cancer Treatment Advances for BRCA1/2 Carriers
Results from a clinical trial of individuals with a BRCA1/2 mutation and pancreatic cancer showed that patients who received a PARP inhibitor (olaparib) for maintenance treatment had almost half the risk of their disease progressing when compared to receiving a placebo.1 In fact, after 2 years, 22.1% of patients who received olaparib had no disease …
Permanent link to this article: https://inheritedcancer.net/3nls2019/
ICARE Newsletter Summer 2019
Prostate Cancer Treatment Advances for BRCA1/2 Carriers
ICARE Newsletter Summer 2019
Prostate Cancer Treatment Advances for BRCA1/2 Carriers
There is now information to suggest that identifying inherited mutations in DNA repair genes, such as BRCA1/2 and other genes, in men with metastatic prostate cancer may open doors for other treatment options. Results of a phase 2 clinical trial among men with metastatic and heavily pre-treated prostate cancer were presented at the American Society …
Permanent link to this article: https://inheritedcancer.net/2nls2019/
ICARE Newsletter Winter 2019
Other Advances in Cancer Treatment Among Cancer Patients with Inherited Disease: Familial Adenomatous Polyposis (FAP)
ICARE Newsletter Winter 2019
Other Advances in Cancer Treatment Among Cancer Patients with Inherited Disease: Familial Adenomatous Polyposis (FAP)
A new drug (sorafenib) showed promising results among patients with desmoid tumors, which are a type of tumor for which patients with Familial Adenomatous Polyposis (FAP) due to APC gene mutations are at risk. These tumors frequently grow and encompass internal organs and can be hard to remove surgically. The newly published research showed that …
Permanent link to this article: https://inheritedcancer.net/9nlw2019/
ICARE Newsletter Winter 2019
Other Advances in Cancer Treatment Among Cancer Patients with Inherited Disease: Lynch Syndrome
ICARE Newsletter Winter 2019
Other Advances in Cancer Treatment Among Cancer Patients with Inherited Disease: Lynch Syndrome
Pertaining to metastatic prostate cancer, recently published data reported 8.1% of men with advanced prostate cancer had evidence of mismatch repair (MMR) mutations in their tumors. These types of mutations are frequently seen in tumors among Lynch syndrome patients. In addition, men with this type of tumor had much poorer survival. Tumors with MMR defects …
Permanent link to this article: https://inheritedcancer.net/7nlw2019/
ICARE Newsletter Winter 2019
Other Advances in Cancer Treatment Among Cancer Patients with Inherited Disease: von Hippel-Lindau (VHL) Disease
ICARE Newsletter Winter 2019
Other Advances in Cancer Treatment Among Cancer Patients with Inherited Disease: von Hippel-Lindau (VHL) Disease
Additional exciting advances include the results of a new drug (pazopanib) to treat an inherited cancer condition called von Hippel-Lindau Disease (VHL), in which patients are predisposed to kidney cancers, pancreatic tumors, and hemangioblastomas (i.e., tumors involving the blood vessels). Study results showed that among 31 patients with VHL, overall response rate with the drug …
Permanent link to this article: https://inheritedcancer.net/8nlw2019/
ICARE Newsletter Winter 2019
New Research and Approvals of PARP Inhibitor Drugs to Treat Prostate Cancer in BRCA Carriers
ICARE Newsletter Winter 2019
New Research and Approvals of PARP Inhibitor Drugs to Treat Prostate Cancer in BRCA Carriers
Treatment among patients with metastatic castration-resistant prostate cancer: A PARP inhibitor (rucaparib) was granted a breakthrough therapy designation in October 2018 for monotherapy (i.e., sole treatment) among men with metastatic castration-resistant prostate cancer (with a BRCA1/2 mutation) who have received at least one prior androgen receptor-directed treatment and taxane-based chemotherapy. This designation was granted based …
Permanent link to this article: https://inheritedcancer.net/3nlw2019/
ICARE Newsletter Winter 2019
New Research and Approvals of PARP Inhibitor Drugs to Treat Breast Cancer in BRCA Carriers
ICARE Newsletter Winter 2019
New Research and Approvals of PARP Inhibitor Drugs to Treat Breast Cancer in BRCA Carriers
Treatment among patients with advanced or metastatic breast cancer: A PARP inhibitor (talazoparib) was approved by the FDA on October 16, 2018 for BRCA carriers with HER2-negative locally advanced or metastatic breast cancer, based on results of the EMBRACA trial outlined in the last ICARE newsletter. Litton JK, et al. N Engl J Med. 2018 …
Permanent link to this article: https://inheritedcancer.net/1nlw2019/
ICARE Newsletter Winter 2019
Expansion of Lynch Syndrome Tumor Spectrum Which May Have Treatment Implications
ICARE Newsletter Winter 2019
Expansion of Lynch Syndrome Tumor Spectrum Which May Have Treatment Implications
Although the Lynch syndrome tumor spectrum is thought to be limited to cancers of the colorectum, endometrium, ovaries, stomach, and a few other cancer types, a recent article suggested there might be a broader tumor spectrum than previously considered. Furthermore, colorectal and endometrial cancers which develop among Lynch syndrome patients frequently are determined on tumor …
Permanent link to this article: https://inheritedcancer.net/6nlw2019/
ICARE Newsletter Winter 2019
Refining Treatment for Aggressive Prostate Cancer in Men with BRCA2
ICARE Newsletter Winter 2019
Refining Treatment for Aggressive Prostate Cancer in Men with BRCA2
A recent study reported that a large proportion of men with aggressive prostate cancer have inherited cancer gene mutations. Specifically, among 400 patients with castration-resistant prostate cancer, 16.2% had a germline mutation in a DNA damage repair gene, including 3% with a BRCA2 mutation. Among BRCA2 carriers, survival was 17.4 months, which was much lower …
Permanent link to this article: https://inheritedcancer.net/5nlw2019/
ICARE Newsletter Winter 2019
New Research and Approvals of PARP Inhibitor Drugs to Treat Ovarian Cancer in BRCA Carriers
ICARE Newsletter Winter 2019
New Research and Approvals of PARP Inhibitor Drugs to Treat Ovarian Cancer in BRCA Carriers
First line maintenance treatment among patients newly diagnosed with advanced ovarian cancer: The results of a trial using a PARP inhibitor (olaparib) as maintenance treatment among ovarian cancer patients with advanced disease, a BRCA mutation, and complete or partial response to platinum-based chemotherapy showed that survival at 3 years was 60% among those who got …
Permanent link to this article: https://inheritedcancer.net/2nlw2019/
ICARE Newsletter Summer 2018
Another PARP-Inhibitor Trial Among BRCA Carriers with Advanced Breast Cancer
ICARE Newsletter Summer 2018
Another PARP-Inhibitor Trial Among BRCA Carriers with Advanced Breast Cancer
In a Phase 3 clinical trial among BRCA carriers with advanced breast cancer, an oral PARP Inhibitor (talazoparib) was compared to standard chemotherapy. Among those who received the PARP inhibitor, risk of disease progression or death was 46% lower, and the response rate was double. Furthermore, the side effect profile, quality-of-life measures, and breast cancer …
Permanent link to this article: https://inheritedcancer.net/8nls2018/
ICARE Newsletter Winter 2018
Advances in the Understanding of Inherited Prostate Cancer
ICARE Newsletter Winter 2018
Advances in the Understanding of Inherited Prostate Cancer
Findings through a recent study reported that inherited cancer gene mutations were present in 8.2% of those with advanced or metastatic prostate cancer, which provides additional support to include this group of men in broader testing, particularly as targeted treatments based on inherited gene mutations becomes increasingly available.1 Another recent study suggested that those with …
Permanent link to this article: https://inheritedcancer.net/10nlw2018/
ICARE Newsletter Winter 2018
Advances in New Treatments for Individuals with Lynch Syndrome
ICARE Newsletter Winter 2018
Advances in New Treatments for Individuals with Lynch Syndrome
A recently published phase II clinical trial investigated the use of a new class of drugs (called PD-1 Inhibitors) in DNA mismatch repair-deficient/ microsatellite instability-high colorectal tumors (which are features seen in the majority of colorectal tumors from individuals with Lynch Syndrome) among patients with metastatic disease.1 Investigators found patients who received two PD-1 Inhibitors …
Permanent link to this article: https://inheritedcancer.net/7nlw2018/
ICARE Newsletter Winter 2018
FDA Approval of PARP Inhibitor (Lynparza) for Treatment of Advanced Breast Cancer
ICARE Newsletter Winter 2018
FDA Approval of PARP Inhibitor (Lynparza) for Treatment of Advanced Breast Cancer
On January 12, 2018, the FDA approved the first PARP Inhibitor (Lynparza) for treatment in patients with advanced breast cancer due to inherited BRCA mutations.1 This drug is already approved for certain BRCA carriers for advanced ovarian cancer. PARP inhibitors were originally developed to target the specific pathway through which cancer develops among those with …
Permanent link to this article: https://inheritedcancer.net/6nlw2018/
ICARE Newsletter Summer 2017
Emerging FDA Approvals of Immunotherapy Among Patients With Metastatic MSI-H Cancers
ICARE Newsletter Summer 2017
Emerging FDA Approvals of Immunotherapy Among Patients With Metastatic MSI-H Cancers
Over the last few years, immunotherapy has emerged as an exciting new class of drugs. As early as 2015, immunotherapy through PD-1 Inhibitors among patients with MSI-H colorectal cancers was shown to be of potential benefit.1 As many individuals with Lynch Syndrome have cancers that are MSI-H and mismatch repair deficient, this class of drugs …
Permanent link to this article: https://inheritedcancer.net/5nls2017/
ICARE Newsletter Summer 2017
New Results of a PARP Inhibitor Study Among BRCA Carriers with Metastatic Breast Cancer
ICARE Newsletter Summer 2017
New Results of a PARP Inhibitor Study Among BRCA Carriers with Metastatic Breast Cancer
Over the last decade, a new class of drugs called “PARP Inhibitors” has been evaluated as a form of targeted treatment among BRCA carriers. Results were recently reported from a Phase 3 clinical trial among BRCA carriers with HER2-negative metastatic breast cancer who received two or less prior chemotherapy regimens for their metastatic disease. Study …
Permanent link to this article: https://inheritedcancer.net/4nls2017/
ICARE Newsletter Winter 2017
Newly Approved PARP-Inhibitor (Rucaparib) to Treat BRCA Carriers with Ovarian Cancer
ICARE Newsletter Winter 2017
Newly Approved PARP-Inhibitor (Rucaparib) to Treat BRCA Carriers with Ovarian Cancer
The FDA just approved another PARP inhibitor, rucaparib, for BRCA carriers with ovarian cancer who have already been treated with two or more chemotherapies. Among those with BRCA-mutant ovarian cancers, 54% had a partial or complete response to the drug with a median duration response of 9.2 months. The agency also approved a companion diagnostic …
Permanent link to this article: https://inheritedcancer.net/6nlw2017/
ICARE Newsletter Winter 2017
New Study Suggesting BRCA1/2 and ATM Are Associated with Aggressive Prostate Cancer
ICARE Newsletter Winter 2017
New Study Suggesting BRCA1/2 and ATM Are Associated with Aggressive Prostate Cancer
Among 799 patients with prostate cancer, the rate of BRCA1/2 mutations was much higher among those who passed away of prostate cancer (6.07%) compared to those with low risk disease (1.44%).1 Among the group that died of prostate cancer, those with BRCA1/2 or ATM mutations passed away at an earlier age and had a shorter …
Permanent link to this article: https://inheritedcancer.net/3nlw2017/
ICARE Newsletter Winter 2017
The Potential Promise of Immunotherapy Targeted to Those with Bi-Allelic Mutations in Lynch Syndrome Genes
ICARE Newsletter Winter 2017
The Potential Promise of Immunotherapy Targeted to Those with Bi-Allelic Mutations in Lynch Syndrome Genes
People with Lynch Syndrome have a non-working Lynch gene (“mutation”), while the other copy of that gene is normal (recognizing that all of these genes come in pairs, with one member of the pair coming from each parent). Over the last few years, there has been an increased realization that some individuals have a mutation …
Permanent link to this article: https://inheritedcancer.net/2nlw2017/
ICARE Newsletter Winter 2016
Potential Use of PARP-Inhibitors Among Men with Prostate Cancer Who Carry a Mutation in BRCA or Other DNA-Repair Gene
ICARE Newsletter Winter 2016
Potential Use of PARP-Inhibitors Among Men with Prostate Cancer Who Carry a Mutation in BRCA or Other DNA-Repair Gene
A recent study published in the New England Journal of Medicine suggests that PARP-Inhibitors may be of potential use in men who are no longer responding to standard treatments and carry either somatic (i.e., tumor) and/or germline (inherited) mutations in DNA-repair genes (i.e., BRCA1/2, ATM, Fanconi Anemia genes and CHEK2).1 Of 49 men with prostate …
Permanent link to this article: https://inheritedcancer.net/4nlw2016/
ICARE Newsletter Summer 2015
Advances in Preventive and Treatment Approaches for Individuals with Lynch Syndrome
ICARE Newsletter Summer 2015
Advances in Preventive and Treatment Approaches for Individuals with Lynch Syndrome
A study of over 1800 individuals with a mutation in one of the Lynch Syndrome genes was recently completed to assess whether aspirin and ibuprofen use may reduce colon cancer risk. Results showed that in those who took aspirin or ibuprofen for between 1 month and 4.9 years, the colon cancer risks were lower than …
Permanent link to this article: https://inheritedcancer.net/6nls2015/
ICARE Newsletter Winter 2015
The First PARP-Inhibitor to Be Approved for Clinical Use in BRCA Carriers
ICARE Newsletter Winter 2015
The First PARP-Inhibitor to Be Approved for Clinical Use in BRCA Carriers
More frequently, cancer drugs are being developed to treat tumors based on their molecular make-up. PARP inhibitors are the first class of drugs specifically developed to treat BRCA-related tumors through targeting the DNA repair pathway. The PARP Inhibitors target this pathway and cause cancer cells to die while healthy cells are spared. Although PARP inhibitors …
Permanent link to this article: https://inheritedcancer.net/2nlw2015/
ICARE Newsletter Winter 2014
Contralateral Mastectomy May Improve Survival in BRCA Mutation Carriers
ICARE Newsletter Winter 2014
Contralateral Mastectomy May Improve Survival in BRCA Mutation Carriers
It has been established that women who carry a germline BRCA mutation face breast cancer risks of 60-70% in their lifetime. After an initial breast cancer diagnosis, these women face a high risk for contralateral breast cancer. Some women with BRCA mutations move forward with contralateral mastectomy when they develop their first breast cancer diagnosis (as …
Permanent link to this article: https://inheritedcancer.net/1nlw2014/
ICARE Newsletter Summer 2013
Male BRCA Carriers Have Poorer Outcomes from Prostate Cancer
ICARE Newsletter Summer 2013
Male BRCA Carriers Have Poorer Outcomes from Prostate Cancer
Over the last few years, a number of studies have suggested that men with germline BRCA mutations (especially BRCA2) have poorer outcomes when they develop prostate cancer. In fact, a recent study of 2019 patients with prostate cancer, including 18 BRCA1 carriers, 61 BRCA2 carriers, and 1940 noncarriers indicated that germline mutations were more frequently …
Permanent link to this article: https://inheritedcancer.net/3nls2013/
ICARE Newsletter Winter 2013
The Selection of Chemotherapy in BRCA Patients with Pancreatic Cancer
ICARE Newsletter Winter 2013
The Selection of Chemotherapy in BRCA Patients with Pancreatic Cancer
Some evidence suggests that individuals with BRCA mutations who develop pancreatic cancer may benefit from specific chemotherapy regimens. In a recent review of this topic, Kim et al reported on a study of 5 patients with BRCA mutations (4 BRCA2 and 1 BRCA1) who were treated with a platinum-based chemotherapy regimen.1 Of these patients, 3 …
Permanent link to this article: https://inheritedcancer.net/1nlw2013/